Novel method for synthesizing ritonavir
A technology of ritonavir and a new method, which is applied in the field of medicinal chemistry, can solve the problems of increasing the difficulty of industrialized large-scale production, harsh reaction conditions, cumbersome operation, etc., and achieve the advantages of large production capacity, production cost and reaction conditions simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
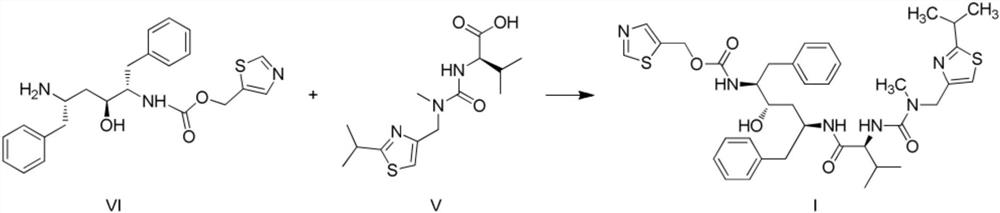
[0025] Embodiment 1 A kind of new method of synthesizing ritonavir
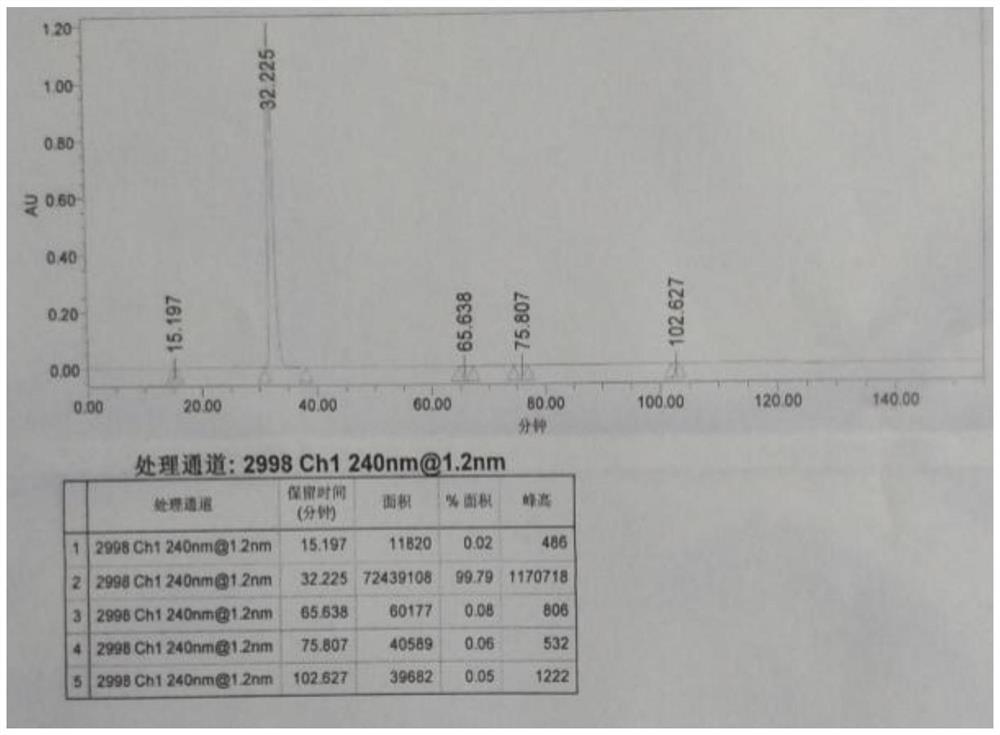
[0026] The reaction kettle R101 is vacuumed, and the nitrogen is evacuated. Under the protection of nitrogen, add 1500kg of dichloromethane and 165kg of intermediate V into the kettle, start stirring and cool down to 5±5°C; add 58.0kg of pivaloyl chloride into the kettle; after adding, Keep the temperature of the reaction kettle R101 at 5±5°C, add 50.0g of triethylamine dropwise into the kettle through the high-level tank V101-I; Add 12.0kg of 4-dimethylaminopyridine (DMAP), stir for 30 minutes, then add the dichloromethane solution of intermediate VI (183.0g dissolved in 500kg); after the addition, the temperature in the kettle is raised to 30±5°C, Stir the reaction for 8-10 hours; take a sample and detect the complete reaction of the intermediate VI by HPLC.
[0027] Post-processing method of ritonavir reaction solution
[0028] Keep the temperature in the reactor at 20±5°C, slowly add 5% sodium hydroxide...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com