A small molecule stat3 inhibitor wz-2-033 and its application in the preparation of drugs for the treatment of breast cancer and gastric cancer
An inhibitor and breast cancer technology, applied in the field of medicine, can solve the problems of low oral bioavailability, unknown mechanism, complex components, etc., and achieve good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Preparation of WZ-2-033
[0045] 2-(2-(4-trifluoromethylphenyl)-7-methoxyimidazol[1,2-a]pyridin-3-yl)-N-(1,1-dioxybenzene[b] The structure of thiophen-6-yl)acetamide (I) is shown below
[0046]
[0047] The specific preparation process is
[0048] Step 1: Preparation of 2-(4-trifluoromethylphenyl)-7-methoxyimidazol[1,2-a]pyridine
[0049]
[0050] Add 600 mg (1 eq) of 2-amino-4'-methoxypyridine and 1 g (1 eq) of 2-bromo-4'-trifluoromethylacetophenone into a clean flask, followed by salt Sodium bicarbonate 660 mg (1.5 equivalents), dissolved in ethanol, stirred at 78°C, heated to reflux for 4 hours, detected by TLC, the reaction was complete, cooled to room temperature, spin-dried the solvent, extracted and separated with ethyl acetate and water, and combined the organic phases , dried over anhydrous sodium sulfate, spin-dried the solvent, and column chromatography (petroleum ether: ethyl acetate = 5:1, V / V) gave 0.9 g of a brownish-yellow solid, with a yield of...
Embodiment 2
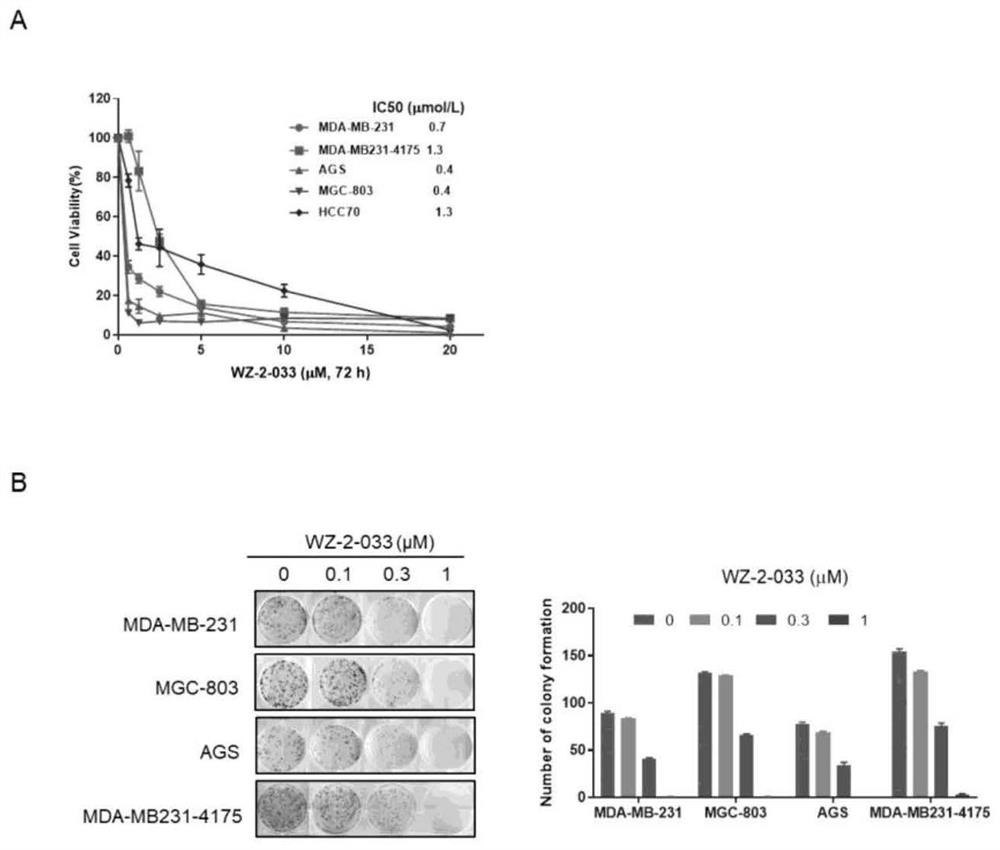
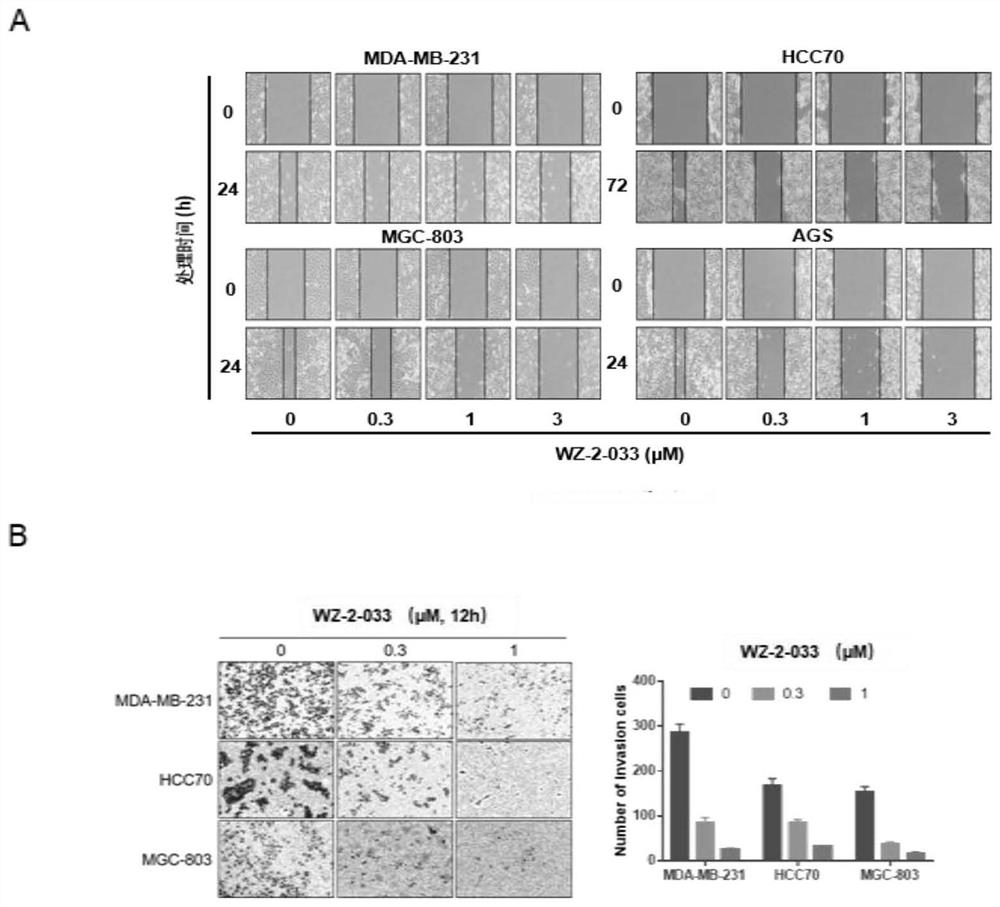
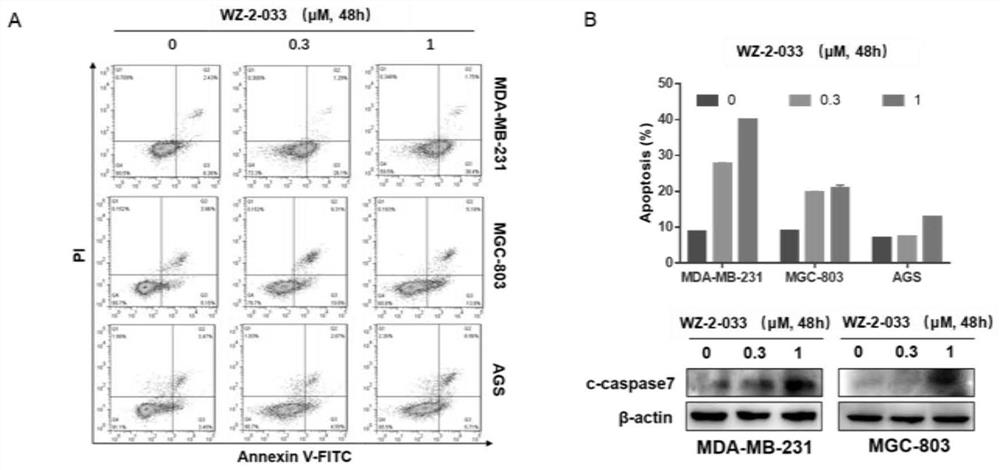
[0065] Example 2: Compound WZ-2-033 small molecule compound inhibits the proliferation of breast cancer cells and gastric cancer cells
[0066] 1. Operation method
[0067] (1) Cell culture
[0068] Human embryonic kidney cells 293 (HEK-293T), breast cancer cells MDA-MB-231, MDA-MB231-4175 and HCC70 gastric cancer cells AGS and MGC-803 used in the present invention are all from the US ATCC cell bank.
[0069] Human embryonic kidney cell 293 (HEK-293T), breast cancer MDA-MB-231 and MDA-MB231-4175 cells were cultured in DMEM medium containing 10% fetal bovine serum and 1% double antibody, gastric cancer cells AGS, MGC- 803 and breast cancer cells HCC70 were cultured in RPMI1640 medium containing 10% fetal bovine serum and 1% double antibody at 37°C, 5% CO 2 in a constant temperature incubator.
[0070] (2) Cell proliferation assay by CCK8 method
[0071] CCK8 reagent contains WST-8 (2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfobenzene)-2H-tetra azole monosodium...
Embodiment 3
[0074] Example 3: Compound WZ-2-033 Small Molecule Compound Inhibits Colony Formation of Breast Cancer and Gastric Cancer Cells
[0075] (1) Cell plate colony formation experiment
[0076] When a single cell proliferates for more than 6 generations in vitro, the cell population composed of its progeny becomes a colony or clone. Each clone contains more than 50 cells with a size between 0.3-1.0mm. The colony formation rate indicates the independent viability of the cells, so the effect of the compound on the viability of cancer cells can be determined by the cell plate colony formation assay.
[0077] Take breast cancer MDA-MB-231 and MDA-MB231-4175 cells in logarithmic growth phase, gastric cancer AGS and MGC-803 cells, digest and resuspend into a single cell suspension, count and inoculate at a density of about 500 cells / well in a six-well plate. After the cells adhered to the wall, set up the control group and the treatment group. The control group was added with the medi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com