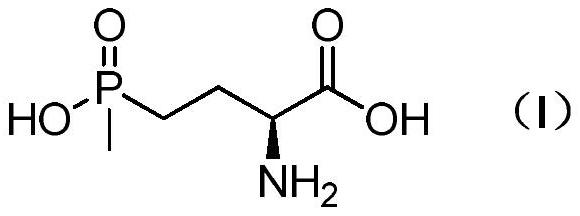
Method for preparing L-glufosinate-ammonium
A technology for glufosinate-ammonium and methylphosphonous dichloride is applied in the field of preparing L-glufosinate-ammonium, can solve the problems of low yield, difficult separation, poor substrate tolerance and the like, and achieves simple steps and easy-to-obtain raw materials. , cost controllable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
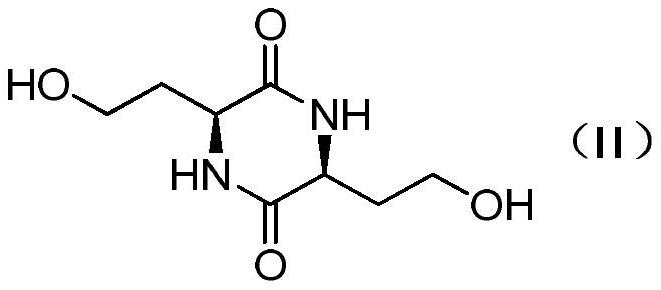
[0031] (1) Preparation of compound 2
[0032]
[0033] Add 140g of L-homoserine (1) (95% ee value) and 250g of ethylene glycol into a three-neck flask, heat up to 150°C and stir for reaction. After 36 hours of reaction, MS detects that the raw materials disappear, cool to 0°C to crystallize and pump Filter to obtain a yellow-brown filter cake, wash with isopropanol three times, and dry to obtain 110 g of brown-yellow solid compound (2), with a yield of 93%.
[0034] The structural identification data of compound 2 are as follows:
[0035] 1 H NMR (400MHz,D 2 O)δ4.26-4.17(m,2H),3.74-3.69(m,4H),2.10-2.02(m,4H); MSCalcd.m / z for C 8 h 14 N 2 o 4 [M+H] + 203.1, found 203.1.
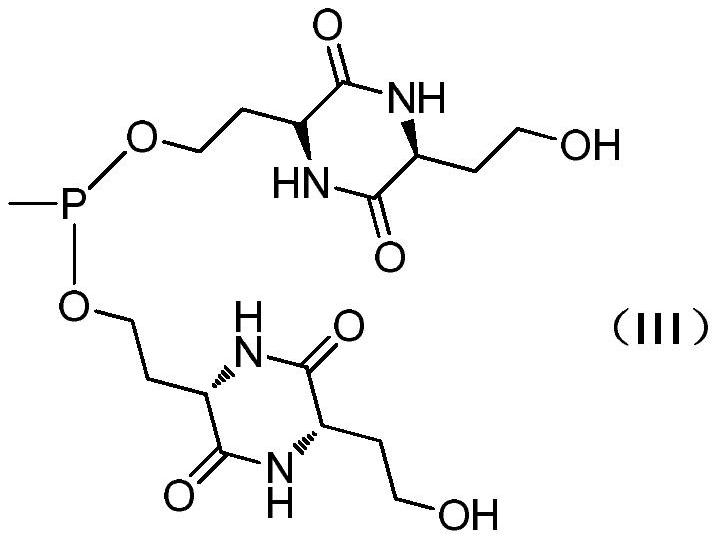
[0036] (2) Preparation of Compound 3
[0037]
[0038] Under nitrogen atmosphere, 110g (0.54mol) of compound (2), 109g (1.08mol) of triethylamine and 200mL of trimethylbenzene were respectively added to the three-necked flask, the temperature was lowered to -20°C, and 28g of methylphosphine dich...
Embodiment 2
[0047] According to the method for Example 1, change the base type in step (2), change the catalyst type and reaction temperature in step (3), the results are shown in Table 1 below.
[0048] Wherein, the molar consumption of different base types in step (2) is 1.08mol, and the catalyst consumption in step (3) is 5g.
[0049] The yield of compound (4) is based on the two-step yield of compound (2), and the yield of L-glufosinate-ammonium is based on the single-step yield of compound (4).
[0050] Table 1
[0051]
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com