Method for preparing multi-morphology nano zinc oxide by solid-phase method
A nano-zinc oxide, multi-morphology technology, applied in the direction of zinc oxide/zinc hydroxide, nanotechnology, nanotechnology, etc., to achieve the effect of easy experimental process, simple process conditions, green and environmental protection yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1. Weigh 11.52g of zinc sulfate heptahydrate (0.04mol) and 3.17g of ammonium bicarbonate (0.04mol) in a ceramic mortar with a diameter of 160mm. At this time, the ratio of zinc sulfate heptahydrate and ammonium bicarbonate is 1:1. Without dispersion medium;
[0042] 2. Manually grind the solid mixture for 30 minutes;
[0043] 3. After the reaction, transfer the mixture in the ceramic mortar to a 50mL centrifuge bottle, add 10mL deionized water, stir the suspension with a glass rod for 3min, then centrifuge the suspension, filter, and continue to add deionized water , repeat the steps of washing and centrifugation 3 times, to remove unreacted complete reactant (zinc sulfate heptahydrate and ammonium bicarbonate) and ammonium sulfate;
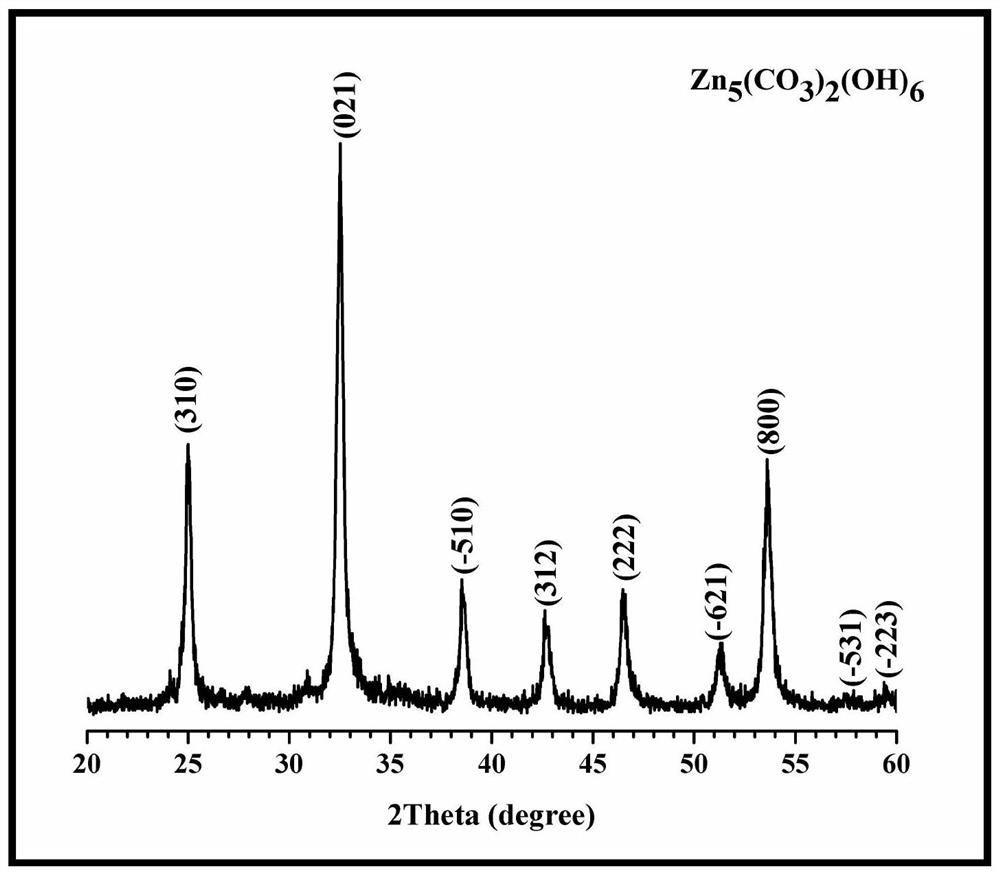
[0044] 4. Dry the centrifuged solid at 60°C for 0.5h to obtain the precursor basic zinc carbonate;
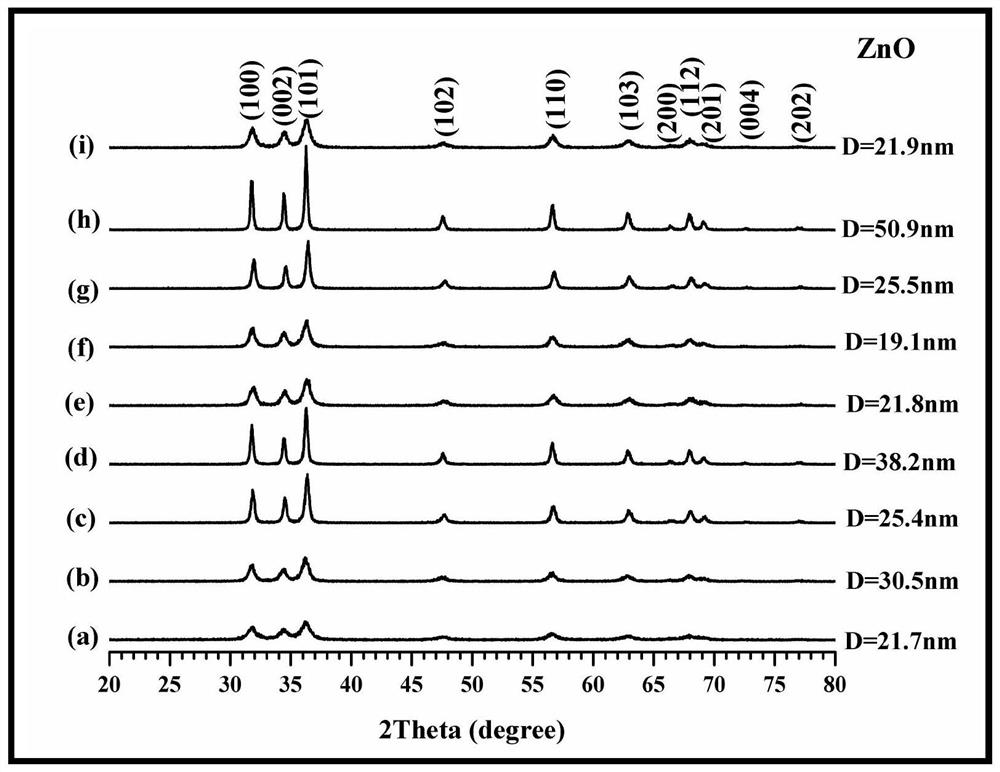
[0045] 5. Put the precursor in a muffle furnace for calcination, the calcination temperature is 300° C., and the calcination time is 1 h...
Embodiment 2
[0049] 1. Weigh 5.76g zinc sulfate heptahydrate (0.02mol) and 3.17g ammonium bicarbonate (0.04mol) in a ceramic mortar with a diameter of 160mm. At this time, the ratio of zinc sulfate heptahydrate and ammonium bicarbonate is 1:2. Add the dispersion medium, the dispersion medium is 5mL ultrapure water;
[0050] 2. Manually grind the solid mixture for 60 minutes;
[0051] 3. After the reaction, transfer the mixture in the ceramic mortar to a 50mL centrifuge bottle, add 20mL of deionized water, stir the suspension with a glass rod for 4min, then centrifuge the suspension, filter, and continue to add deionized water , repeat the steps of washing and centrifugation 4 times, to remove unreacted complete reactant (zinc sulfate heptahydrate and ammonium bicarbonate) and ammonium sulfate;
[0052] 4. Dry the centrifuged solid at 70°C for 1 hour to obtain the precursor basic zinc carbonate;
[0053] 5. Put the precursor in a muffle furnace for calcination. The calcination temperature...
Embodiment 3
[0056] 1. Weigh 3.75g of zinc sulfate heptahydrate (0.013mol) and 3.17g of ammonium bicarbonate (0.04mol) in a ceramic mortar with a diameter of 160mm. At this time, the ratio of zinc sulfate heptahydrate and ammonium bicarbonate is 1:3. Add the dispersion medium, the dispersion medium is 5mL absolute ethanol;
[0057] 2. Manually grind the solid mixture for 90 minutes;
[0058] 3. After the reaction, transfer the mixture in the ceramic mortar to a 50mL centrifuge bottle, add 30mL deionized water, stir the suspension with a glass rod for 5min, then centrifuge the suspension, filter, and continue to add deionized water , repeat the steps of washing and centrifugation 5 times, to remove unreacted complete reactant (zinc sulfate heptahydrate and ammonium bicarbonate) and ammonium sulfate;
[0059] 4. Dry the centrifuged solid at 80°C for 2 hours to obtain the precursor basic zinc carbonate;
[0060] 5. Put the precursor in a muffle furnace for calcination. The calcination tempe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com