Bifidobacterium animalis NX-6 and application thereof in preparation of lipid-lowering and weight-losing medicines
An animal bifidobacteria, NX-6 technology, applied in the field of microorganisms, can solve the problems of insufficient development of probiotics' blood lipid-lowering function, imperfect functional evaluation path, and few probiotics, etc., to prevent or treat cardiovascular diseases, Good weight and length gain, the effect of improving body mass index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Isolation, Identification and Preservation of Novel Animal Bifidobacterium NX-6
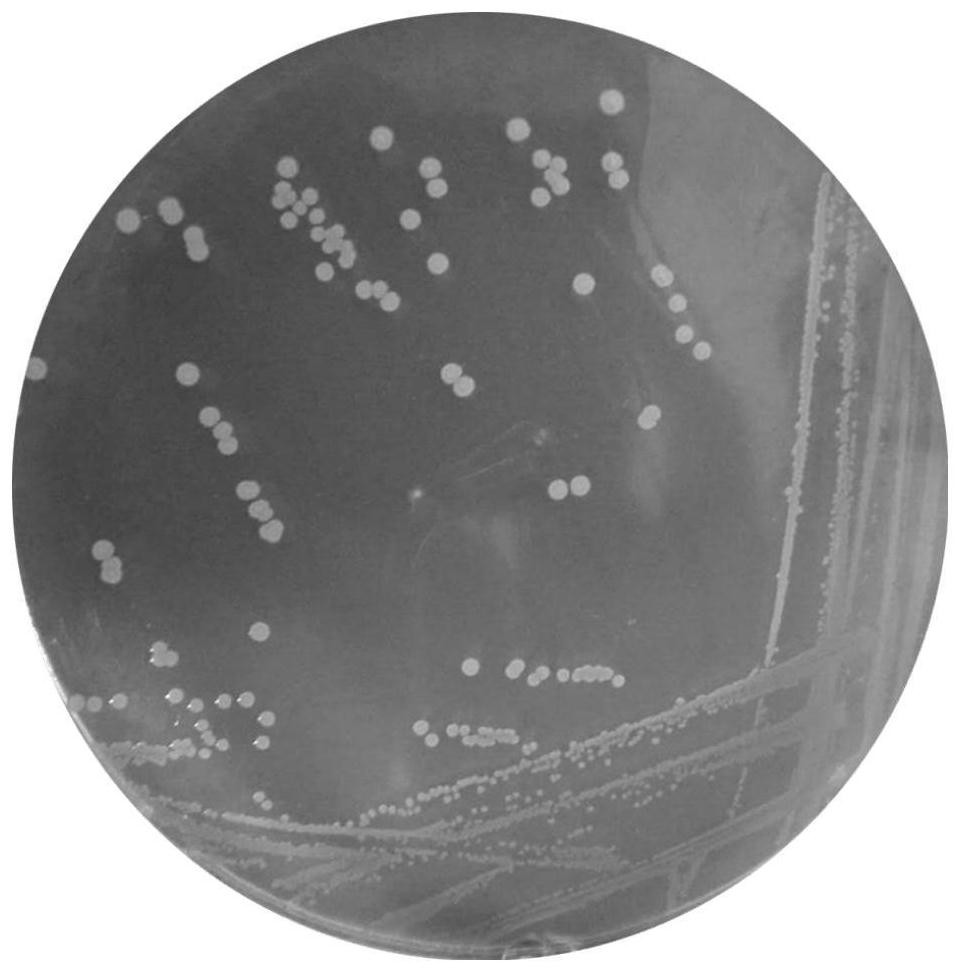
[0028] (1) Isolation: After the fecal samples were serially diluted, they were respectively inoculated in BS solid medium, cultured anaerobically at 37°C for 48h-72h, and the single colony on the plate was picked and streaked for three generations to obtain the purified strain. Inoculate the single clone obtained by streaking the third generation into BS liquid medium, culture anaerobically at 37°C for 12-16h, add 40% glycerol, mix the bacterial liquid and glycerol in equal volumes, and store in a -80°C refrigerator.
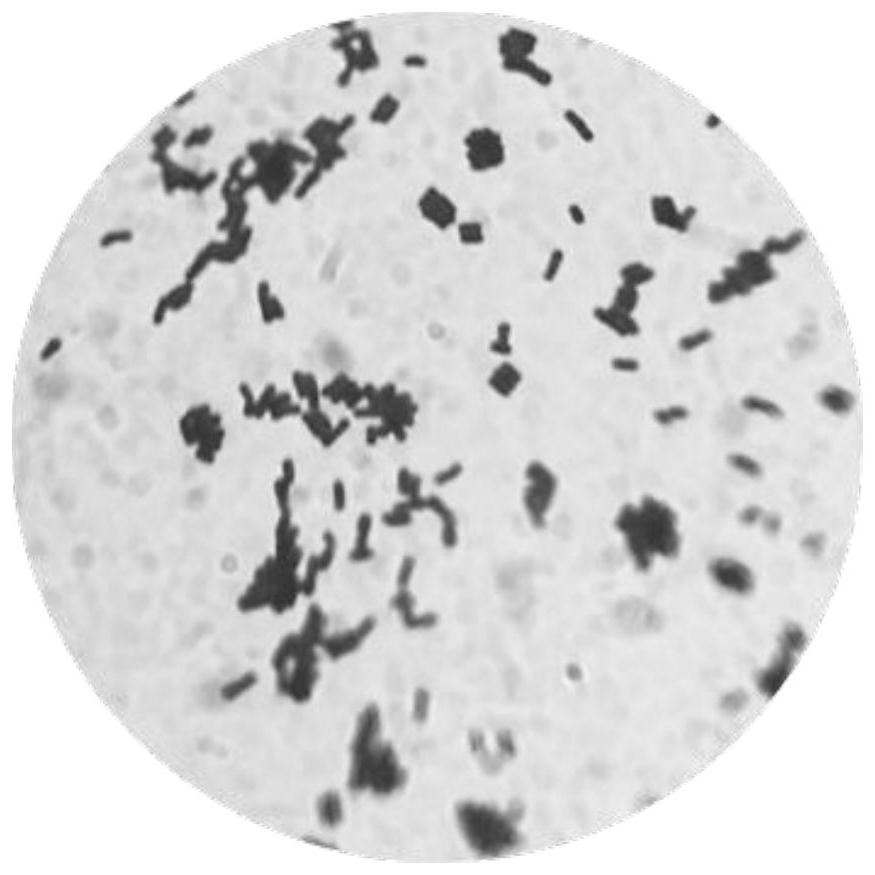
[0029] (2) Morphological identification of strains: Enzyme contact test was carried out on the screened strains to observe whether there were bubbles. Gram-stained strains were checked with an oil microscope, Gram-positive strains were purple, and Gram-negative strains were red. Bifidobacteria can be observed in Y-type and V-type characteristics.
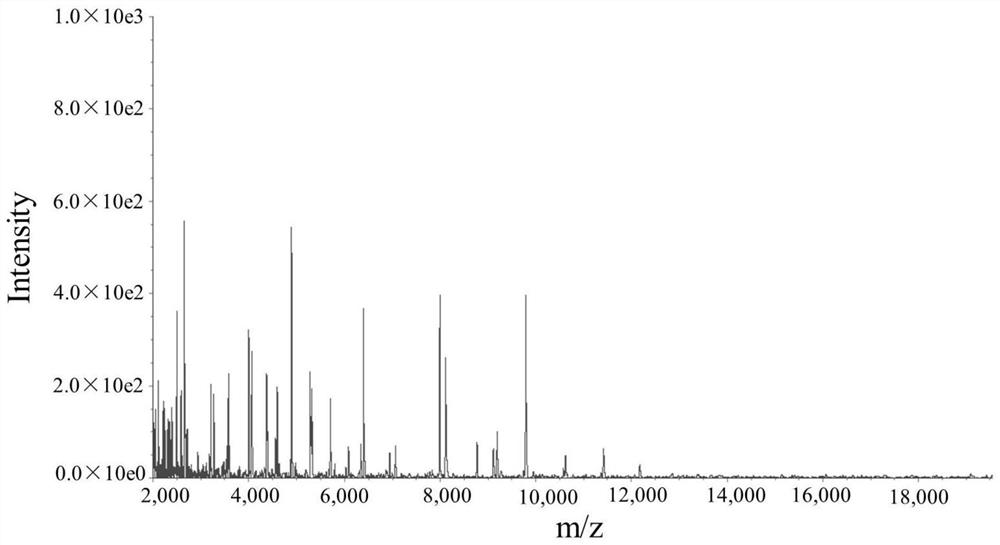
[0030] (3) Rap...
Embodiment 2
[0037] The preparation of embodiment 2 novel bifidobacterium animalis NX-6 fermentation supernatant (extracellular secretion), bacterium suspension (thalline) and cell disruptor supernatant (intracellular substance)
[0038] The new type of bifidobacterium animalis NX-6 was activated and cultured and inoculated in BS liquid medium. After culturing at 37°C for 15 hours, the concentration of fermentation bacteria was adjusted to 10 9 CFU / mL, centrifuged at 4°C, 6000r / min for 10min to obtain the culture supernatant and cell pellet, the supernatant was filtered through a 0.22μm filter membrane to obtain the fermentation supernatant (extracellular secretion); the cell pellet was filtered through PBS twice After the first wash, the cells were resuspended with PBS, and the cell concentration was adjusted to 10 9 CFU / mL to obtain bacterial suspension (bacteria); process the bacterial suspension in an ice bath with an ultrasonic breaker, work for 3s, interval 8s, ultrasonic break for 1...
Embodiment 3
[0039] Embodiment 3 zebrafish rearing
[0040] Lovastatin was purchased from Shanghai Luyuan Biotechnology Co., Ltd., and common feed and high-cholesterol feed were purchased from Nantong Trophy Feed Technology Co., Ltd.
[0041] Under the microscope, the normal wild-type AB line zebrafish (5dpf) was selected and divided into normal group (blank control group), model group (high cholesterol), positive control group (lovastatin), samples (not inactivated and inactivated: Bacterial suspension, fermentation supernatant, cell crush supernatant) intervention group, 200 pieces in each group. The zebrafish in the normal group were fed with common feed (40 mg each time); the zebrafish in the model group were fed with a high-cholesterol feed (40 mg each); the zebrafish in the positive control group were fed with a high-cholesterol feed+lovastatin (high cholesterol feed and lovastatin) Statin was mixed in a weight ratio of 19:1, 40 mg each time); the zebrafish in the sample interventio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com