A kind of perovskite carbon dioxide electroreduction catalyst and preparation method thereof
A perovskite type, carbon dioxide technology, applied in the direction of physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, etc., can solve problems such as inappropriateness, achieve lower levels, lower The effect of simple cost and preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
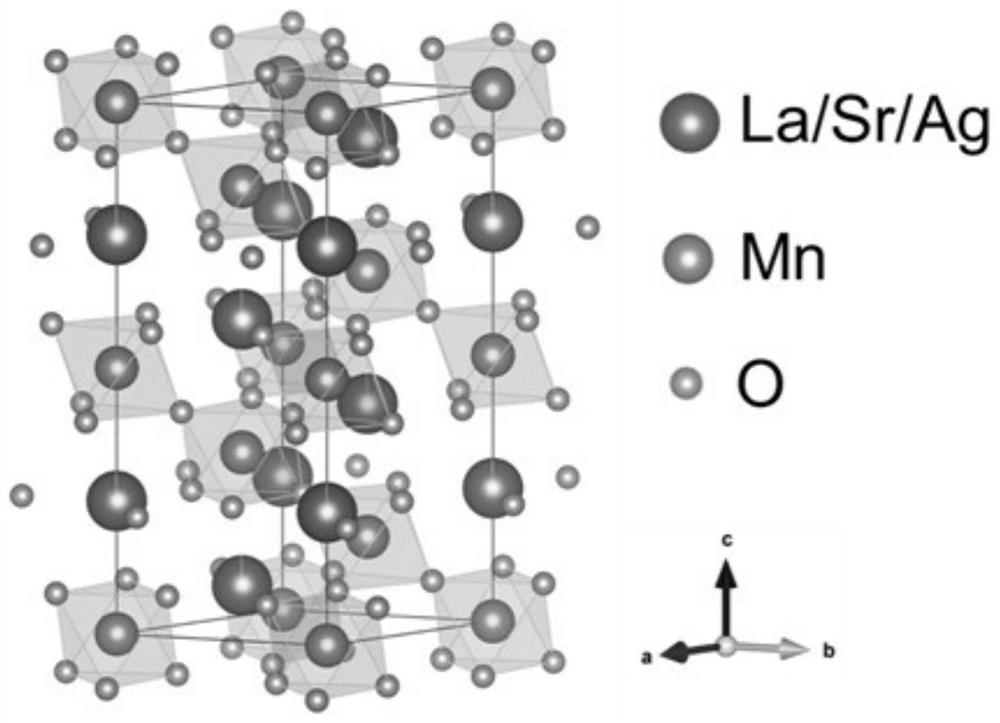
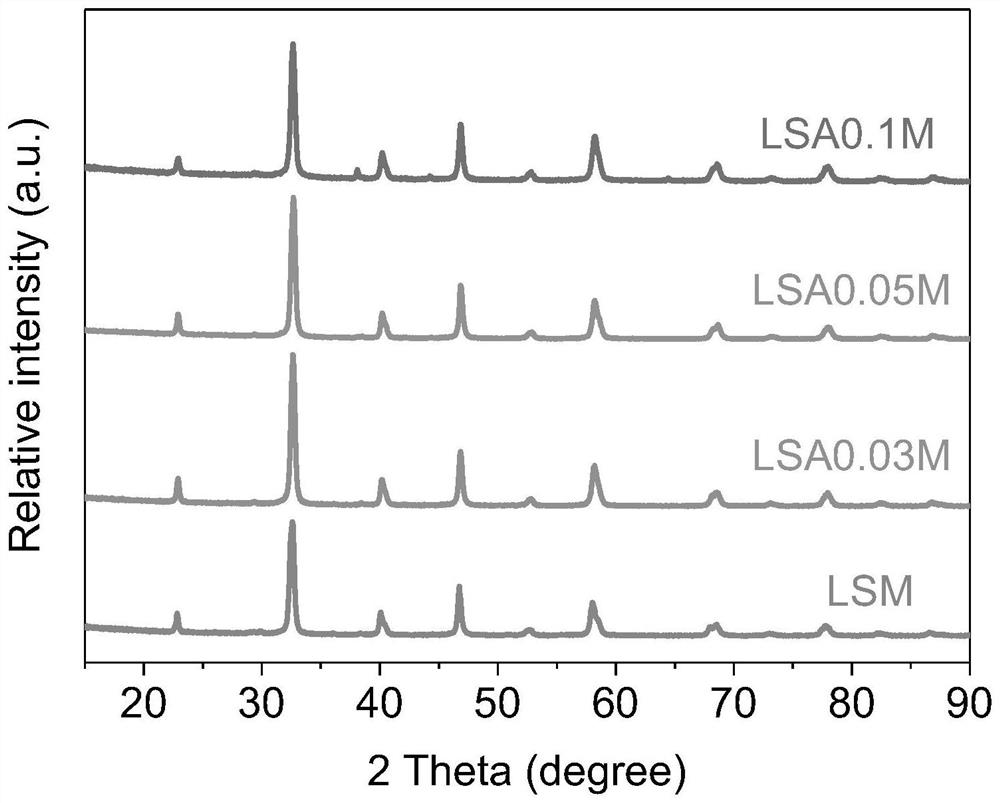
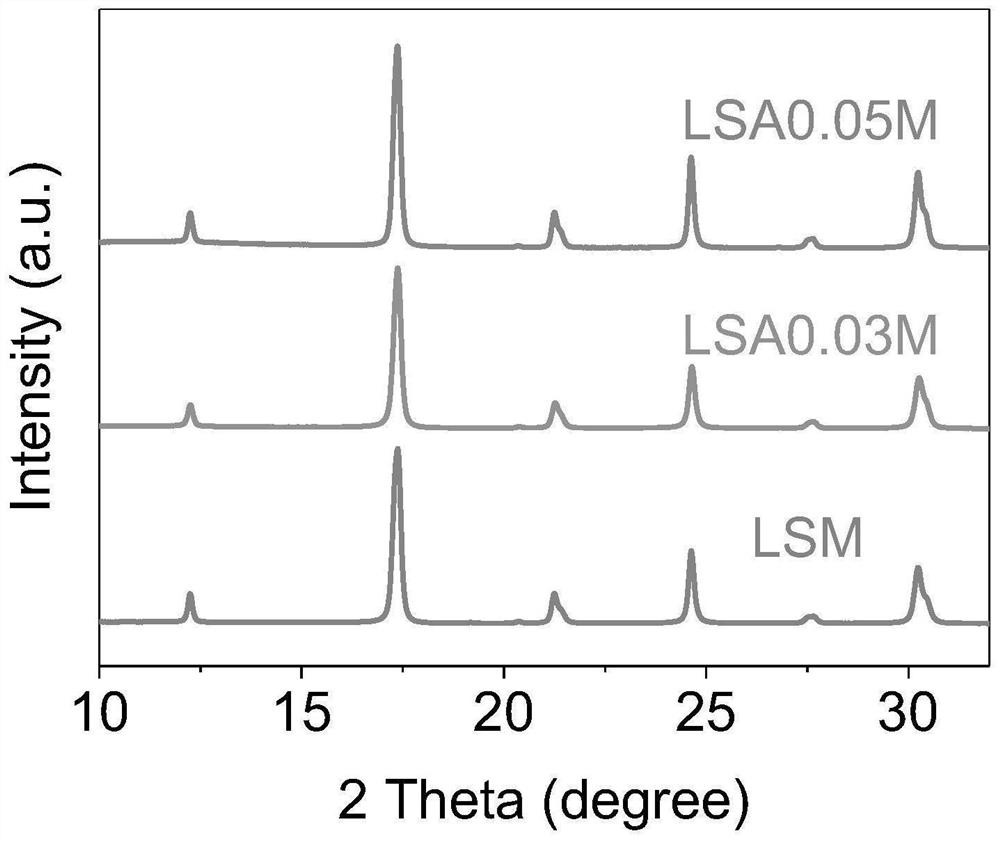
[0039] Example 1 (La 0.8 Sr 0.2 ) 0.95 Ag 0.05 MnO 3 Preparation of catalyst
[0040] Weigh 3.291g La (NO 3 ) 3 ·6H 2 O, 0.402g Sr(NO 3 ) 2 , 0.085g AgNO 3 , 2.451g of manganese acetate tetrahydrate and 8.5g of citric acid monohydrate, pour them into a beaker, and add an appropriate amount of deionized water to stir on a magnetic stirring table to mix them evenly until clear. 5.85g of ethylenediaminetetraacetic acid and 20mL of ammonia water were mixed into a clear solution and poured into the beaker described above to prepare a precursor solution. The solution was heated with continuous stirring at 90°C-100°C until the solution turned into a gel. The gelatinous mixture was sent to a 200°C electric blast drying oven for drying for 8 hours to obtain a black fluffy solid precursor. A part of the precursor was taken and placed in a tube furnace, an oxygen atmosphere was introduced, the temperature was raised to 800°C at a heating rate of 5°C / min and kept for 5h. Afte...
Embodiment 2
[0041] Example 2 (La 0.8 Sr 0.2 ) 0.97 Ag 0.03 MnO 3 Preparation of catalyst
[0042] Weigh 3.361g La (NO 3 ) 3 ·6H 2 O, 0.411g Sr(NO 3 ) 2 , 0.051g AgNO 3 , 2.451g of manganese acetate tetrahydrate and 8.5g of citric acid monohydrate, pour them into a beaker, and add an appropriate amount of deionized water to stir on a magnetic stirring table to mix them evenly until clear. 5.85g of ethylenediaminetetraacetic acid and 20mL of ammonia water were mixed into a clear solution and poured into the beaker described above to prepare a precursor solution. The solution was heated with continuous stirring at 90°C-100°C until the solution turned into a gel. The gelatinous mixture was sent to a 200°C electric blast drying oven for drying for 8 hours to obtain a black fluffy solid precursor. A part of the precursor was taken and placed in a tube furnace, an oxygen atmosphere was introduced, the temperature was raised to 800°C at a heating rate of 5°C / min and kept for 5h. Afte...
Embodiment 3
[0043] Example 3 (La 0.8 Sr 0.2 ) 0.9 Ag 0.1 MnO 3 Preparation of catalyst
[0044] Weigh 3.118g La (NO 3 ) 3 ·6H 2 O, 0.381g Sr(NO 3 ) 2 , 0.170g AgNO 3 , 2.451g of manganese acetate tetrahydrate and 8.5g of citric acid monohydrate, pour them into a beaker, and add an appropriate amount of deionized water to stir on a magnetic stirring table to mix them evenly until clear. 5.85g of ethylenediaminetetraacetic acid and 20mL of ammonia water were mixed into a clear solution and poured into the beaker described above to prepare a precursor solution. The solution was heated with continuous stirring at 90°C-100°C until the solution turned into a gel. The gelatinous mixture was sent to a 200°C electric blast drying oven for drying for 8 hours to obtain a black fluffy solid precursor. A part of the precursor was taken and placed in a tube furnace, fed with an oxygen atmosphere, raised to 800°C at a heating rate of 5°C / min and kept for 5h. After natural cooling, take out ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com