Novel series of immune agonists
A technology of agonists and compounds, applied in the interdisciplinary field of medicinal chemistry and immunology, which can solve the problems of weak activity, limited clinical practicability, and insufficient immunotherapy effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
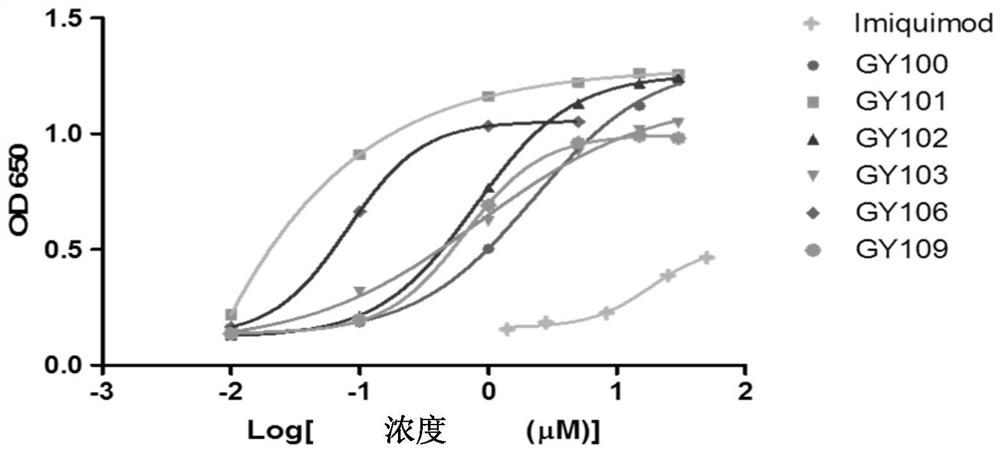
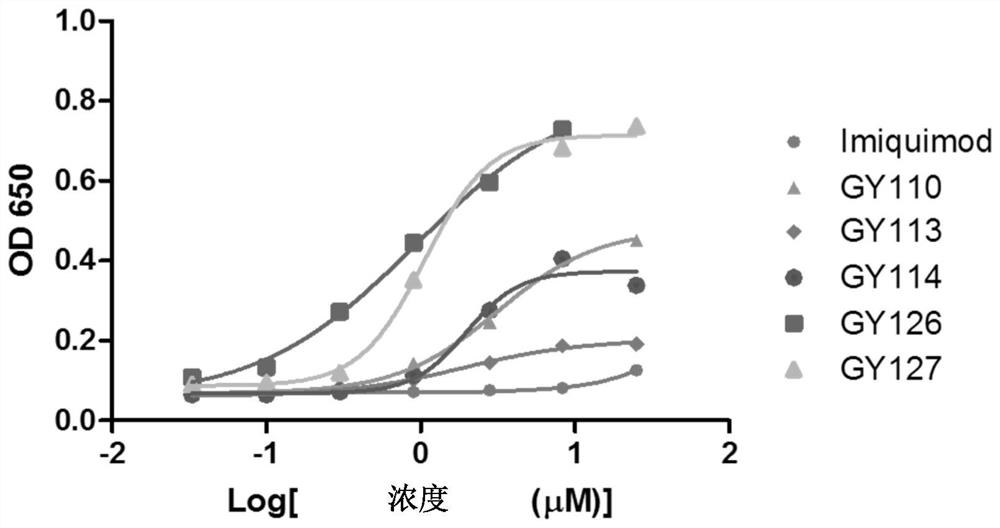
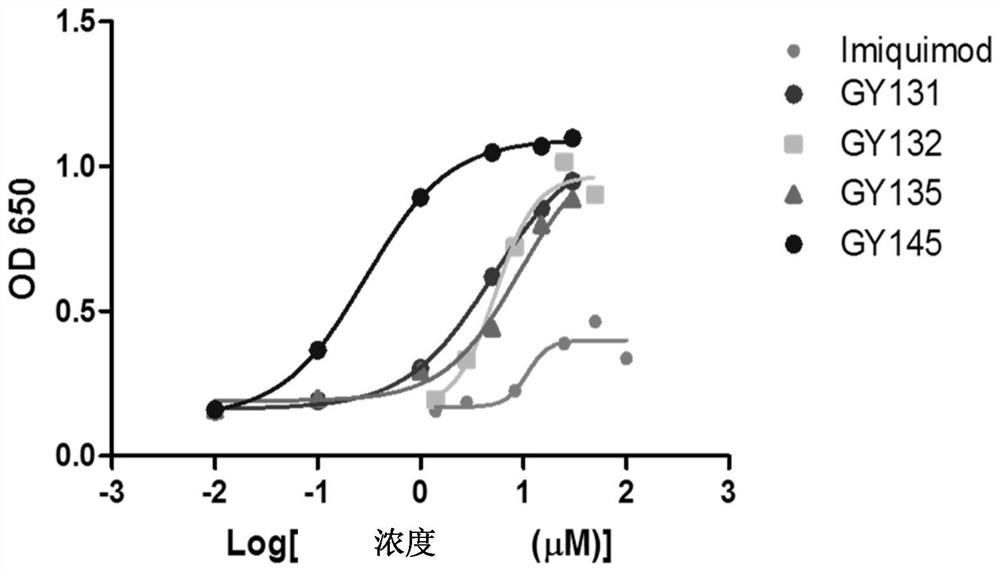
[0405] Example 1 Detection of TLR7 activation of GY series compounds or drugs (HEK-Blue TM Detection)
[0406] Take HEK-Blue in the logarithmic growth phase TM For hTLR7 cells (purchased from InvivoGen), discard the growth medium (Gibco, C11995500BT, Invivo Gen, ant-nr), take an appropriate amount of 37°C PBS (Hyclone, SH30256.01) and gently wash the cells twice, discard the PBS . Add 2-5mL 37°C PBS, incubate for 1-2min, scrape the cells with a cell scraper and blow gently to disperse them into a single-cell suspension. Count cells and calculate cell concentration using a hemocytometer, using HEK-Blue TM Dectetion solution (purchased from Invivo Gen) to adjust the cell suspension to 2.5 × 10 4 / 180 μL per well for cell plating on 96-well cell culture plates. Stimulate the HEK-Blue TM For hTLR7 cells, three replicate wells were set for each concentration. Incubate for 6-16 hours at 37°C and 5% carbon dioxide. After the incubation, the absorbance value was read at a wave...
Embodiment 2
[0412] Example 2 GY series compound immune cell inflammatory factor activation experiment
[0413] The stimulating effect of GY series compounds or drugs on mouse spleen lymphocytes was detected by ELISA.
[0414] 2-1. Acquisition of mouse spleen lymphocytes
[0415] Take 6-week-old Balb / c mice, kill them by cervical dislocation, take out their spleens under aseptic conditions, use a 1 mL sterile syringe and a 200-mesh cell filter, and put them in 4 mL of mouse lymphocyte separation medium (Daktronics, 7211011 ), quickly grind the spleen to disperse single cells. Transfer the cell homogenate to a 15mL centrifuge tube, slowly add 1mL RPMI 1640 medium (Hyclone, SH30809.01), and use density gradient centrifugation (800×g, 30min) to separate spleen lymphocytes, wash and lyse the erythrocytes, A dispersed spleen lymphocyte suspension was obtained. Count the cells with a hemocytometer and calculate the cell concentration, and adjust the cell suspension to 1×10 with RPMI 1640 comp...
Embodiment 3
[0431] Example 3 CCK8 method to detect the growth inhibitory effect or proliferation effect of GY series compounds or drugs on cells
[0432] Mouse breast cancer 4T1 cells in the logarithmic growth phase (KG338) were washed with PBS, digested with 0.25% trypsin (containing EDTA), digested with DMEM complete medium, centrifuged, and resuspended. Make 4T1 cells a single cell suspension. Count the cells with a hemocytometer and calculate the cell concentration. Use DMEM complete medium to adjust the cell suspension to the target concentration, according to 4×10 3 / 100 μL per well for cell plating on 96-well cell culture plates. After the cells adhered to the wall, the 4T1 cells were stimulated according to the designed GY series compound or drug concentration gradient: 3 replicate wells were set for each concentration. The 96-well cell culture plate that was seeded with 4T1 cells and finished adding drugs was placed in a cell culture incubator, and cultured at 37°C and 5% carbo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com