Method for producing avian influenza vaccine by adopting MDCK cell line and product of avian influenza vaccine
A cell line, avian influenza technology, applied in the field of vaccine preparation, can solve the problems of decreased virus yield, unsuitable for large-scale culture, and unable to meet the needs of virus vaccine production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] A kind of method adopting MDCK cell line to produce bird flu vaccine comprises the steps:
[0048] (1) Cell culture: Mix DMEM, F12 and RPMI1640 evenly in a ratio of 3:1:1, use this as the basal medium and add 10% FBS, revive and culture the adherent MDCK cells, and wait until the cells grow to dense cells After layering, digested with 0.25% EDTA-trypsin, passed 5 times; gradually reduced the proportion of DMEM-F12-RPMI1640 medium containing 10% FBS, increased the proportion of serum-free DMEM-F12-RPMI1640 medium, so that the serum content gradually changed from 10 % reduced to 0%;
[0049] After digesting the well-growing MDCK cells adapted to serum-free culture with 0.25% EDTA-trypsin, culture on a shaking table at a speed of 40r / min; after the cell growth rate is stable, subculture, gradually expand the culture, and gradually increase the speed of the shaking table , until acclimatized to a stable proliferation of serum-free full suspension culture MDCK cell line;
...
Embodiment 2
[0058] A kind of method adopting MDCK cell line to produce bird flu vaccine comprises the steps:
[0059] (1) Cell culture: Mix DMEM, F12 and RPMI1640 evenly in a ratio of 3:1:1, use this as the basal medium and add 10% FBS, revive and culture the adherent MDCK cells, and wait until the cells grow to dense cells After layering, digested with 0.25% EDTA-trypsin, passed 5 times; gradually reduced the proportion of DMEM-F12-RPMI1640 medium containing 10% FBS, increased the proportion of serum-free DMEM-F12-RPMI1640 medium, so that the serum content gradually changed from 10 % reduced to 0%;
[0060] After digesting the well-growing MDCK cells adapted to serum-free culture with 0.25% EDTA-trypsin, culture on a shaking table at a speed of 40r / min; after the cell growth rate is stable, subculture, gradually expand the culture, and gradually increase the speed of the shaking table , until acclimatized to a stable proliferation of serum-free full suspension culture MDCK cell line;
...
Embodiment 3
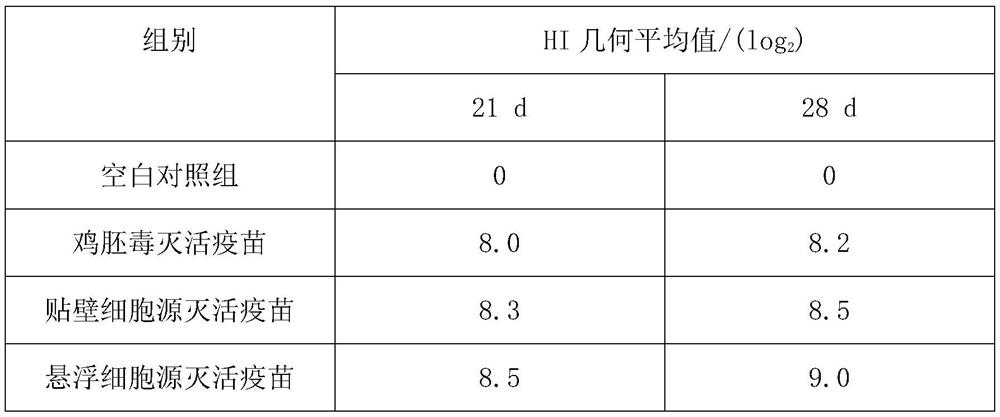
[0069] This embodiment illustrates the inspection and measurement and effect experiment of the vaccine product prepared in Example 2.
[0070] 1. Inspection of semi-finished products:
[0071] Sterility test: According to the "Quality Standards for Veterinary Biological Products of the People's Republic of China" appendix 301, sterile growth.
[0072] Inactivation test: After inactivation, samples were taken from the virus inactivation solution under aseptic conditions, and 10 10-day-old SPF chicken embryos were inoculated via the allantoic cavity route, 0.1ml / embryo, incubated at 37°C, and observed in eggs every day, 120 There should be no death within 1 hour. If there is death, the allantoic fluid should have no hemagglutination value.
[0073] 2. Finished product inspection
[0074] Properties: The appearance is white emulsion, and the dosage form is oil-in-water-in-oil type.
[0075] Stability: Stored at 37°C for 21 days or centrifuged at 3000r / min for 15 minutes, the e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com