Deuterated loxapine drug and preparation method thereof
A loxapine and deuterated technology, applied in the field of loxapine drug synthesis, can solve the problems of multiple steps of resources, use of toxic, waste of environment, etc., and achieve the effects of reducing cost, reducing pollution and waste, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Using amoxapine as a raw material to synthesize N-CD 3 loxapine
[0039]
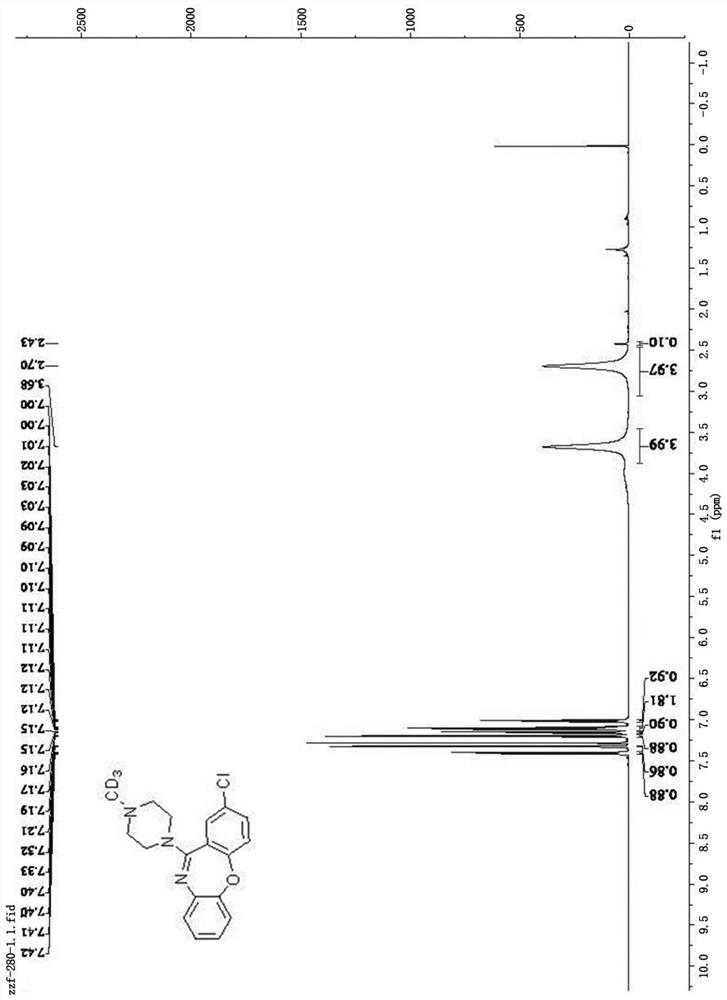
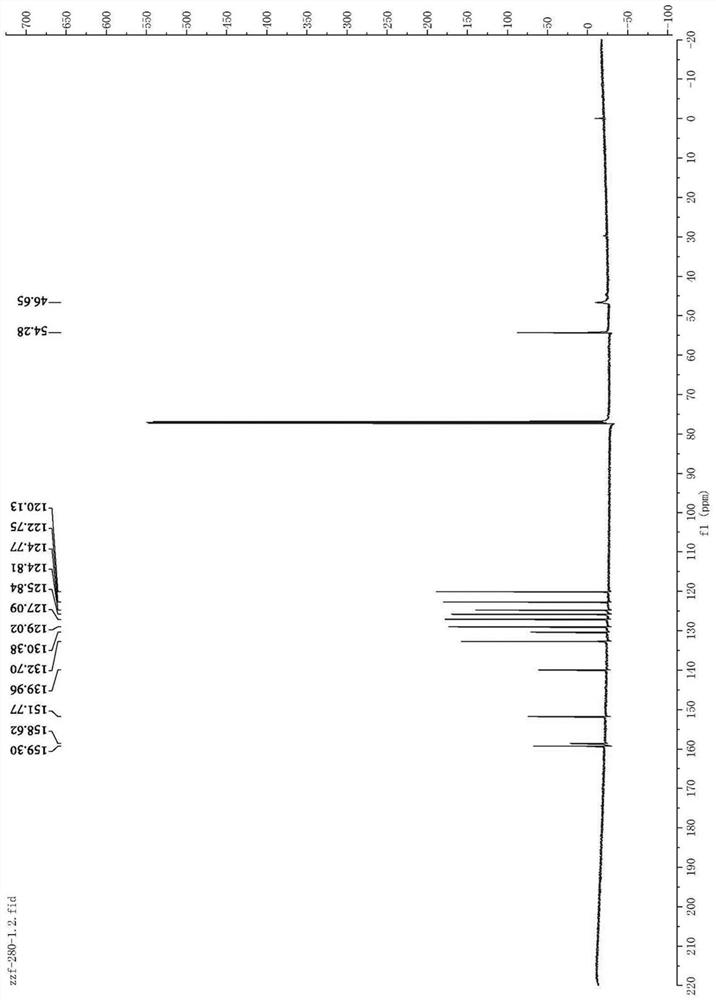
[0040] Weigh 0.4mmol of amoxapine, 25.0mg of Pd / KPCN photocatalyst and 40mg of aluminum chloride into a 5mL reaction flask, add 2mL of anhydrous acetonitrile, and add deuterium water / d 4 - A mixed solution of deuterated methanol (1.0mL / 0.6mL), the reaction system was replaced by an argon protection state, and then the reaction bottle was placed under a 420nm light source for 24 hours of light reaction, and the light source was removed after the reaction. Filter through celite, then wash with 5.0 mL CH 2 Cl 2 After extraction, the extract was dried over anhydrous sodium sulfate and concentrated to obtain a colorless liquid. The solvent is removed by rotary evaporation, and then the pure target product is obtained by column chromatography (developing solvent: dichloromethane / methanol), such as Figure 1-2 shown by 1 HNMR, C-NMR and other tests confirm the structure, the yield is ...
Embodiment 2
[0041] Example 2: Using amoxapine as a raw material to synthesize N-CD 2 H loxapine
[0042]
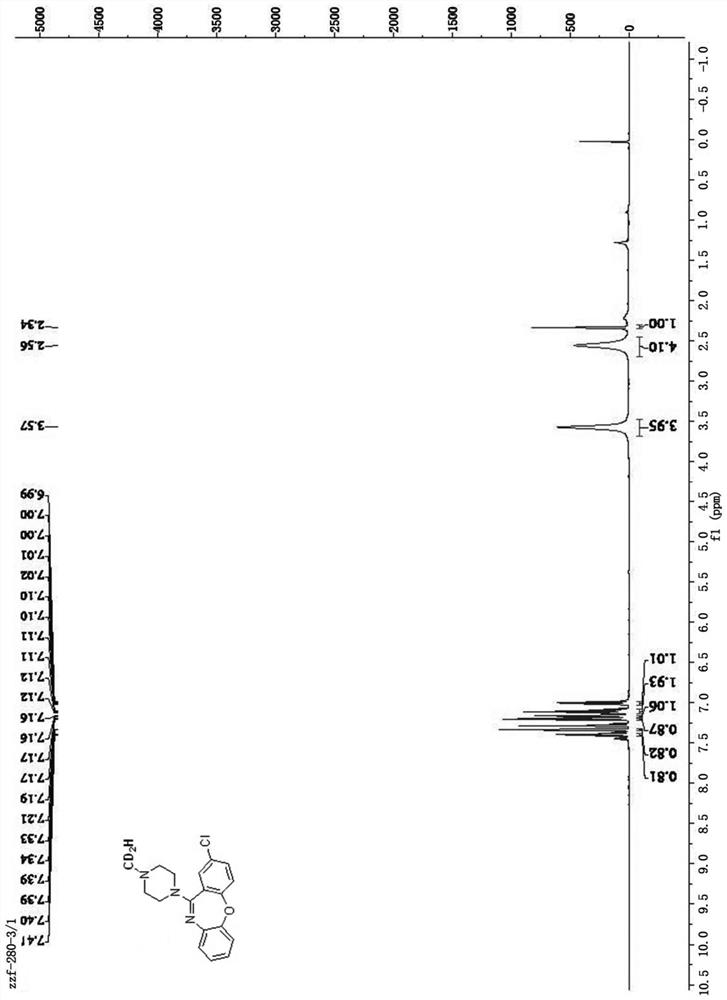
[0043] Weigh 0.4mmol of amoxapine, 25.0mg of Pd / KPCN photocatalyst and 40mg of aluminum chloride into a 5mL reaction flask, add 2mL of anhydrous acetonitrile, and add water / d 4 - A mixed solution of deuterated methanol (1.0mL / 0.6mL), the reaction system was replaced by an argon protection state, and then the reaction bottle was placed under a 420nm light source for 24 hours of light reaction, and the light source was removed after the reaction. Filter through celite, then wash with 5.0 mL CH 2 Cl 2 After extraction, the extract was dried over anhydrous sodium sulfate and concentrated to obtain a colorless liquid. The solvent is removed by rotary evaporation, and then the pure target product is obtained by column chromatography (developing solvent: dichloromethane / methanol), such as image 3 shown by 1 HNMR, C-NMR and other tests confirm the structure, the yield is 87%, and th...
Embodiment 3
[0044] Example 3: Using amoxapine as a raw material to synthesize N-CDH 2 loxapine
[0045]
[0046] Weigh 0.4mmol of amoxapine, 25.0mg of Pd / KPCN photocatalyst and 40mg of aluminum chloride into a 5mL reaction flask, add 2mL of anhydrous acetonitrile, and add deuterium water / d 1 - A mixed solution of deuterated methanol (1.0mL / 0.6mL), the reaction system was replaced by an argon protection state, and then the reaction bottle was placed under a 420nm light source for 24 hours of light reaction, and the light source was removed after the reaction. Filter through celite, then wash with 5.0 mL CH 2 Cl 2 After extraction, the extract was dried over anhydrous sodium sulfate and concentrated to obtain a colorless liquid. The solvent is removed by rotary evaporation, and then the pure target product is obtained by column chromatography (developing solvent: dichloromethane / methanol), such as Figure 4 shown by 1 HNMR, C-NMR and other tests confirm the structure, the yield is 8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com