Chromone skeleton-containing polycyclic compound as well as preparation method and application thereof
A technology for polycyclic compounds and compounds, applied in the field of preparation of polycyclic compounds containing chromone skeleton, can solve the problems of unreported synthetic methods and high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
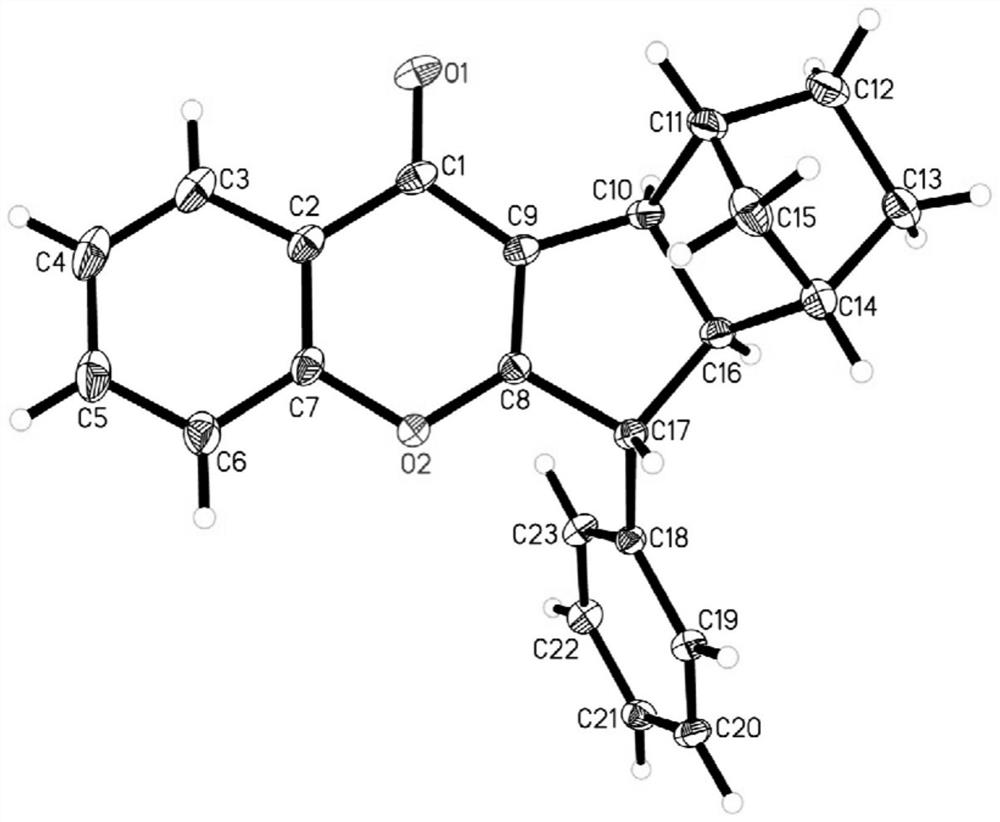
[0057] 3-iodochromone (0.2mmol), benzyl bromide (0.22mmol), norbornene (0.8mmol, 75.3mg), palladium acetate (0.02mmol, 4.5mg), three (2-methylphenyl) phosphorus (0.04mmol, 12.2mg), potassium phosphate (0.4mmol, 84.9mg), mesitylene (1.2mL) and acetonitrile (0.8mL) were added to a 4mL reaction flask under nitrogen at 100°C, and reacted at 100°C for 24h, After the reaction is finished, cool to room temperature, and the resulting reaction solution is subjected to column chromatography (the eluent is obtained by mixing ethyl acetate and petroleum ether according to a volume ratio of 50:1–20:1), and the obtained reaction solution is obtained by column chromatography. The solvent was removed from the solution to obtain 23 mg of a yellow solid (4a), and 20 mg of a light yellow solid mixture of 4a and 4a', with an overall yield of 65%. The diastereoselectivity was determined to be 86:14, and the melting point was 158.0-159.3°C.
[0058] The above-mentioned yellow solid was characterize...
Embodiment 2
[0065] The 3-iodochromone in Example 1 was replaced by 3-iodo-5-methoxychromone, and the other conditions were the same as in Example 1 to finally obtain a light green solid with a yield of 43.0 mg and a yield of 60%. , its diastereoselectivity was detected to be 99:1, and its melting point was 175.9~177.1°C.
[0066] The above-mentioned light green solid was characterized by NMR, and the specific result data are as follows:
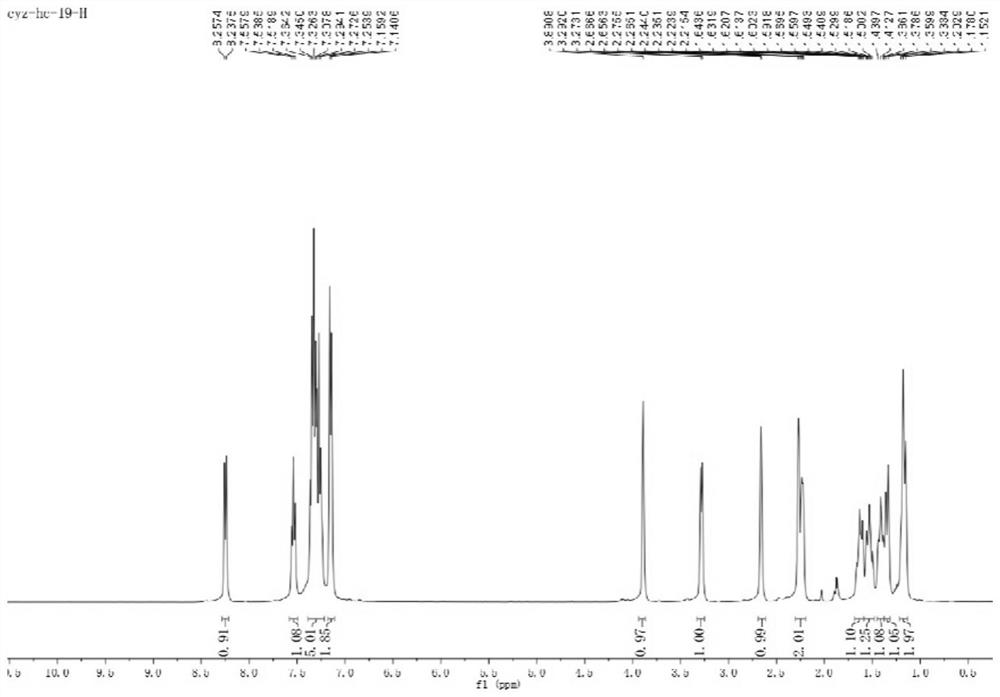
[0067] 1 H NMR (400MHz, CDCl 3 )δ7.40(t,J=8.4Hz,1H),7.34–7.28(m,2H),7.26–7.21(m,1H),7.15–7.11(m,2H),6.86–6.82(m,1H) ,6.77–6.73(m,1H),3.95(s,3H),3.84–3.81(m,1H),3.25–3.15(m,1H),2.68(d,J=4.2Hz,1H),2.24(d ,J=4.2Hz,1H),2.20–2.15(m,1H),1.66–1.56(m,1H),1.54–1.46(m,1H),1.42–1.31(m,2H),1.18–1.10(m ,2H);
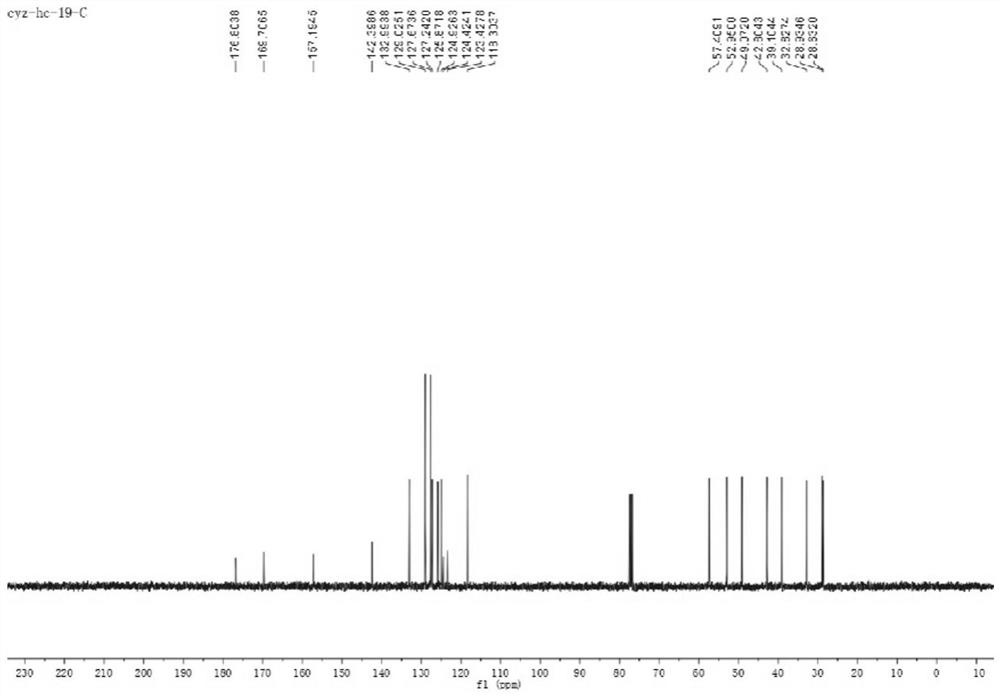
[0068] 13 C NMR (100MHz, CDCl 3 )δ176.9, 167.1, 160.4, 159.6, 142.5, 133.0, 129.0, 127.7, 127.2, 124.7, 115.2, 110.6, 106.4, 57.1, 56.6, 53.2, 49.3, 42.9, 39.0, 32.8, 28.9, 28.I7. ):calcd.for C 24 h 23 o 3 [M+H] + 359.1642; found 359.1645.
[0069]...
Embodiment 3
[0072] The 3-iodochromone in Example 1 was replaced by 3-iodo-6-methylchromone, and other conditions were the same as in Example 1 to finally obtain a yellow solid with a yield of 52.0 mg and a yield of 76%. Its diastereoselectivity was detected to be 81:19, and its melting point was 169.5-171.3°C.
[0073] The above green solid was characterized by NMR, and the specific result data are as follows:
[0074] 1H NMR (400MHz, CDCl 3 )δ8.03–8.00(m,1H),7.36–7.29(m,3H),7.27–7.24(m,1H),7.18(d,J=8.5Hz,1H),7.15–7.13(m,2H) ,3.89–3.85(m,1H),3.29–3.25(m,1H),2.65(d,J=4.2Hz,1H),2.42(s,3H),2.26(d,J=4.3Hz,1H), 2.24–2.19(m,1H),1.66–1.58(m,1H),1.57–1.47(m,1H),1.44–1.31(m,2H),1.22–1.11(m,2H);
[0075] 13 C NMR (100MHz, CDCl 3 )δ176.9, 169.6, 155.6, 142.6, 134.9, 134.2, 129.0, 127.7, 127.2, 125.3, 124.1, 123.3, 118.1, 57.5, 53.0, 49.1, 42.9, 39.2, 32.9, 29.0, 28.7, 21.0 ):calcd.for C 24 h 23 o 2 [M+H] + 343.1693; found 343.1697.
[0076] After analysis, it can be seen that the structu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com