Conjugate containing alpha-galactosylceramide analogue and carbohydrate antigen as well as preparation method and application of conjugate
A galactosylceramide and conjugate technology, applied in the field of anti-tumor sugar vaccine development, can solve the problems of uncertain glycoprotein vaccine coupling site, complex composition, unstable coupling rate and the like, and achieve high yield , easy operation, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] This example is a conjugate containing an α-galactosylceramide analogue and a carbohydrate antigen provided by the present invention, and its structural formula is shown in the following formula (III):
[0076]
[0077] The preparation method of the above-mentioned conjugate containing α-galactosylceramide analogue and carbohydrate antigen comprises the following steps:
[0078] (1) Compound 1 and Compound 2 are dissolved in an organic solvent, and a condensing agent is added to react to obtain Compound 3; the reaction formula to obtain Compound 3 is shown in the following formula:
[0079]
[0080] The specific operation of step (1) is: dichloromethane solution (80.0mL) dissolves (compound 1) Fmoc-L-threonine (4.5g, 13.1mmol) and (compound 2) 2-[2-(2-propane Alkynyloxy)ethoxy]ethylamine (2.2g, 15.7mmol), N,N'-dicyclohexylcarbodiimide (3.0g, 14.4mmol) and 1-hydroxybenzotriazole ( 0.7g, 1.3mol), remove the ice bath, return to room temperature, stir and react at ro...
experiment example 1
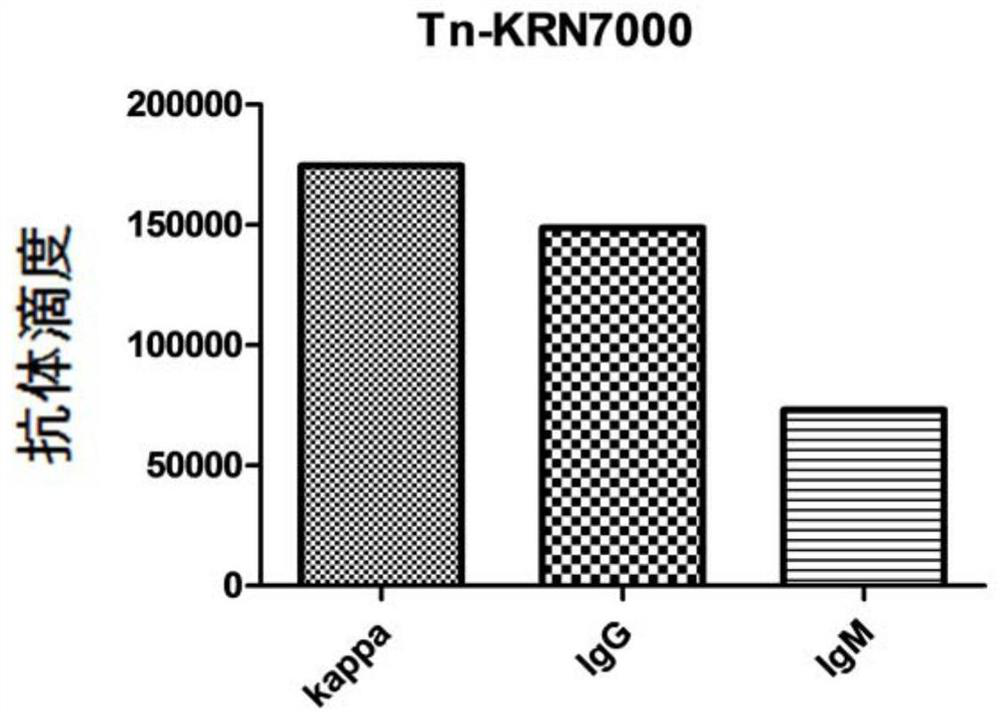
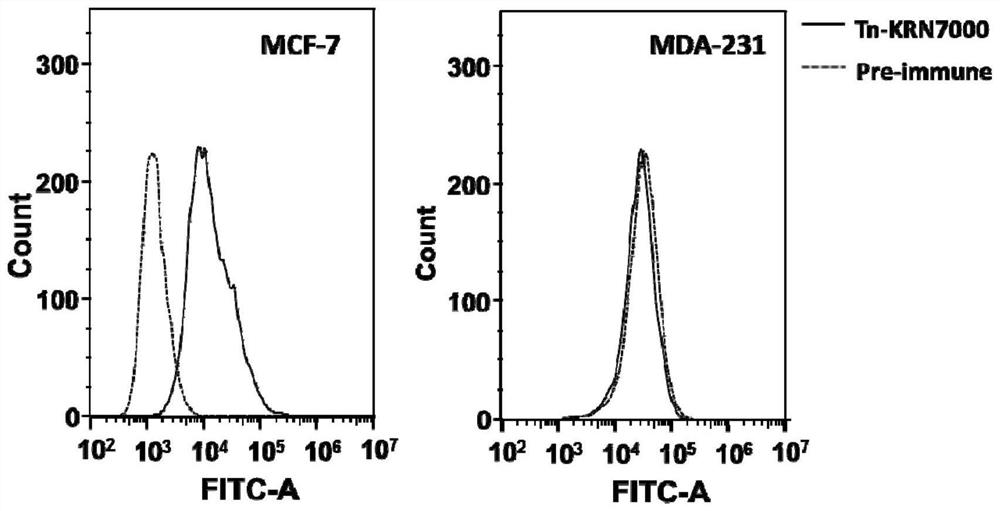
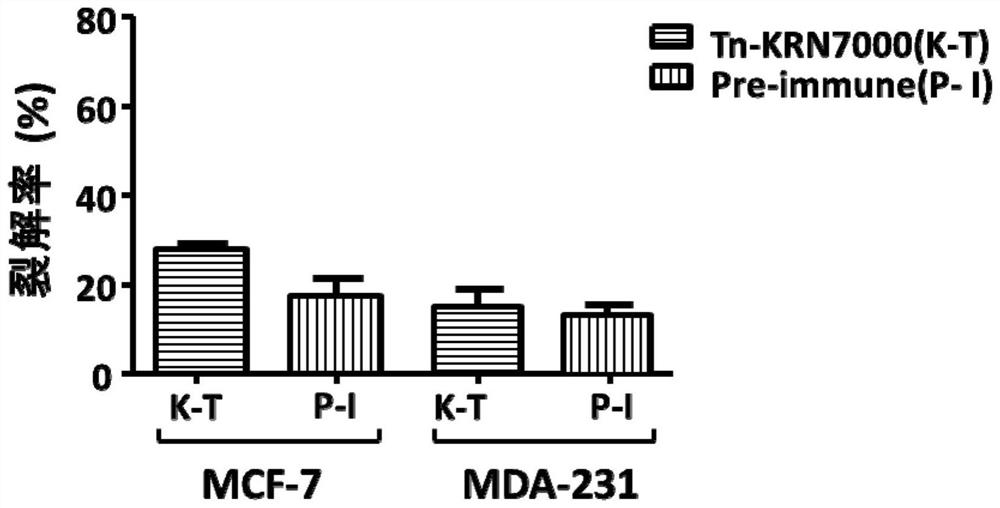
[0106] In this experimental example 1, mice were immunized with the conjugate (fully synthetic sugar vaccine) prepared in Example 1, and its immune effect was preliminarily evaluated by ELSA experiment, and the antibody serum was proved to be able to specifically recognize by fluorescence activated cell sorting (FACS). Tumor cells (MCF-7), and antibody-mediated complementation-dependent cytotoxicity (CDC) experiments showed that antibody serum has the ability to kill tumor cells mediated by complement.
[0107] 1. ELISA immunoassay
[0108] 1) Immunization of mice:
[0109] Take 6 C57BL / 6 mice aged 6-8 weeks. After the sugar vaccine was prepared into liposomes, the immune test was carried out by subcutaneous injection in mice. One initial immunization and three booster immunization schemes were used to inject the prepared vaccines on days 0, 14, 21, and 28 respectively. The injection volume is 0.1mL; on the 38th day, 0.1mL to 0.2mL of blood is collected from each mouse, plac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com