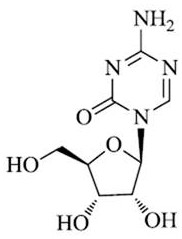
Azacitidine freeze-dried preparation for injection and preparation method thereof
A technology of azacitidine and freeze-dried preparations is applied in the field of stable freeze-dried preparations of azacitidine for injection and its preparation, which can solve the problem of affecting the bioequivalence of reconstituted suspension and affecting the crystal grains in the pre-freezing stage. It can solve problems such as path and path, and achieve the effect of solving the peak-to-valley fluctuation of blood drug concentration, solving frequent dosing and high encapsulation efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
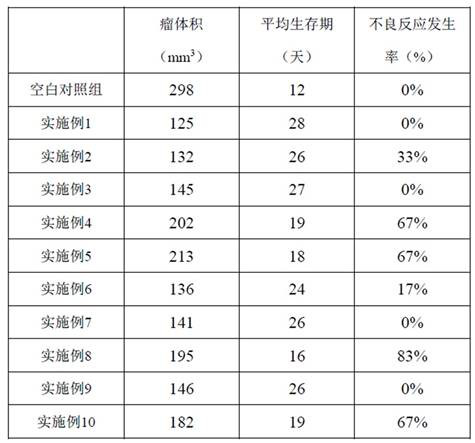
Embodiment 1
[0036] The azacitidine of 2 weight parts, the gelatin of 1 weight part are dissolved in the water of 6 volume parts, dissolve and mix as internal water (W 1 ) phase; 5 parts by weight of polylactic acid-glycolic acid (the molar ratio of lactic acid and glycolic acid in polylactic acid-glycolic acid is 75 / 25, molecular weight 6000) is dissolved in 60 parts by volume of dichloromethane as oil (O) Phase; 5 parts by weight of polyvinyl alcohol was dissolved in 600 parts of water for injection at 75°C as (W 2 ) phase; the W 1 Slowly drop the phase into the O phase, emulsify at a high speed of 18000r / min for about 3~5min, and add W 2 In the phase, 18000r / min high-speed oscillation emulsification for 3~5min, 500r / min for 3h to evaporate dichloromethane, 12000r / min high-speed centrifugation, wash 5 times with water for injection, sub-package, freeze-dry to obtain.
[0037] The freeze-drying method is:
[0038] The lyophilizer is cooled to -40°C in advance, and the filled samples ar...
Embodiment 2
[0040] The azacitidine of 2 weight parts, the gelatin of 1 weight part are dissolved in the water of 6 volume parts, dissolve and mix as internal water (W 1 ) phase; 3 parts by weight of polylactic acid-glycolic acid (the molar ratio of lactic acid and glycolic acid in polylactic acid-glycolic acid is 75 / 25, molecular weight 6000) is dissolved in 60 parts by volume of dichloromethane as oil (O) Phase; 5 parts by weight of polyvinyl alcohol was dissolved in 600 parts of water for injection at 75°C as (W 2 ) phase; the W 1 Slowly drop the phase into the O phase, emulsify at a high speed of 18000r / min for about 3~5min, and add W 2In the phase, 18000r / min high-speed oscillation emulsification for 3~5min, 500r / min for 3h to evaporate dichloromethane, 12000r / min high-speed centrifugation, wash 5 times with water for injection, sub-package, freeze-dry to obtain. The freeze-drying method is the same as in Example 1.
Embodiment 3
[0042] The azacitidine of 2 weight parts, the gelatin of 1 weight part are dissolved in the water of 6 volume parts, dissolve and mix as inner water (W 1 ) phase; 6 parts by weight of polylactic acid-glycolic acid (the molar ratio of lactic acid and glycolic acid in polylactic acid-glycolic acid is 75 / 25, molecular weight 6000) is dissolved in 60 parts by volume of dichloromethane as oil (O) Phase; 10 parts by weight of polyvinyl alcohol was dissolved in 1000 parts of water for injection at 75°C as (W 2 ) phase; the W 1 Slowly drop the phase into the O phase, emulsify at a high speed of 18000r / min for about 3~5min, and add W 2 In the phase, 18000r / min high-speed oscillation emulsification for 3~5min, 500r / min for 3h to evaporate dichloromethane, 12000r / min high-speed centrifugation, wash 5 times with water for injection, sub-package, freeze-dry to obtain. The freeze-drying method is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com