Manganese-doped Cs3Cu2I5 halide scintillator with high light yield
A halide and manganese doping technology, applied in the direction of luminescent materials, chemical instruments and methods, etc., can solve the problems of high price, low detection limit, unfavorable commercial application, etc., achieve high stability, simple preparation method, and environmental friendliness Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
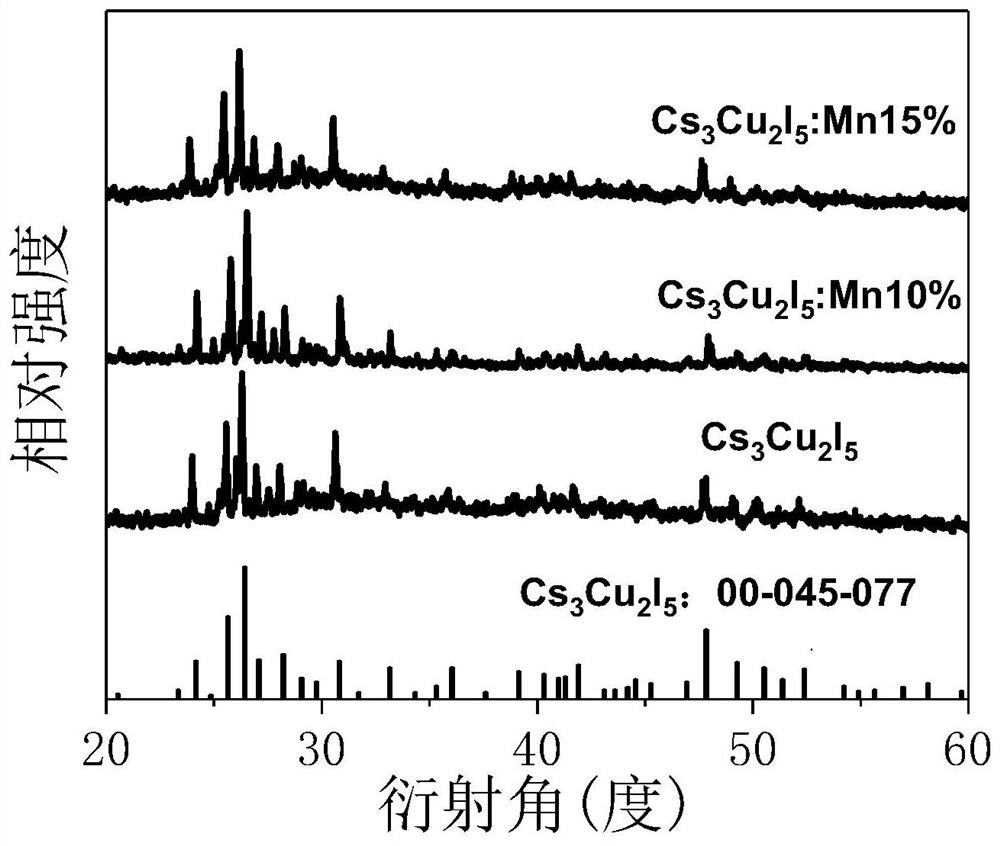
[0031] According to the chemical formula Cs 3 Cu 2 I 5According to the stoichiometric ratio of each element in CsI: 0.003mol and CuI: 0.002mol, weigh a certain amount of samples respectively, pour them into a mortar or ball mill jar and grind them thoroughly to obtain a uniform mixture; package the obtained mixture placed in a vacuum quartz tube and placed in a muffle furnace, and calcined at 450 °C for 6 h; after the reaction was completed and naturally cooled to room temperature, the calcined product was taken out and ground to obtain a light white Cs 3 Cu 2 I 5 powder; finally, the above powder was vacuum-dried at 80°C for 24 h to obtain the final scintillator material.
Embodiment 2
[0033] According to the chemical formula Cs 3 Cu 2 I 5 : Mn 2+ The stoichiometric ratio of each element, when the molar weight of manganese ion is 0.03 mol, the molar weight of CsI and CuI is 0.003 mol and 0.019 mol respectively, according to the above molar weight ratio, take a certain amount of sample, pour into mortar or The mixture was fully ground in a ball mill jar to obtain a uniform mixture; the obtained mixture was packaged in a vacuum quartz tube, placed in a muffle furnace, and calcined at 450 °C for 6 h; after the reaction was completed and naturally cooled to room temperature, the Calcination of the product and grinding it yielded pale yellow Cs 3 Cu 2 I 5 : Mn 2+ powder; finally, the above powder was vacuum-dried at 80°C for 24 h to obtain the final scintillator material.
Embodiment 3
[0035] According to the chemical formula Cs 3 Cu 2 I 5 : Mn 2+ The stoichiometric ratio of each element, when the molar weight of manganese ion is 0.05 mol, the molar weight of CsI and CuI is 0.003 mol and 0.019 mol respectively, according to the above molar weight ratio, take a certain amount of sample, pour into mortar or The mixture was fully ground in a ball mill jar to obtain a uniform mixture; the obtained mixture was packaged in a vacuum quartz tube, placed in a muffle furnace, and calcined at 450 °C for 6 h; after the reaction was completed and naturally cooled to room temperature, the Calcination of the product and grinding it yielded pale yellow Cs 3 Cu 2 I 5 : Mn 2+ powder; finally, the above powder was vacuum-dried at 80°C for 24 h to obtain the final scintillator material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com