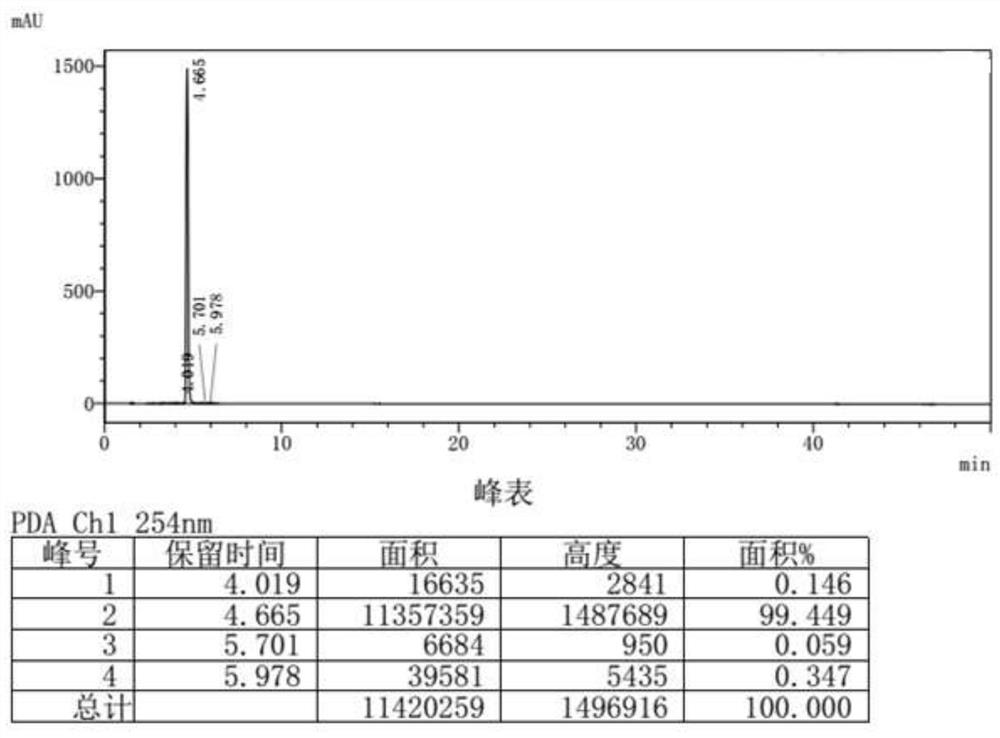
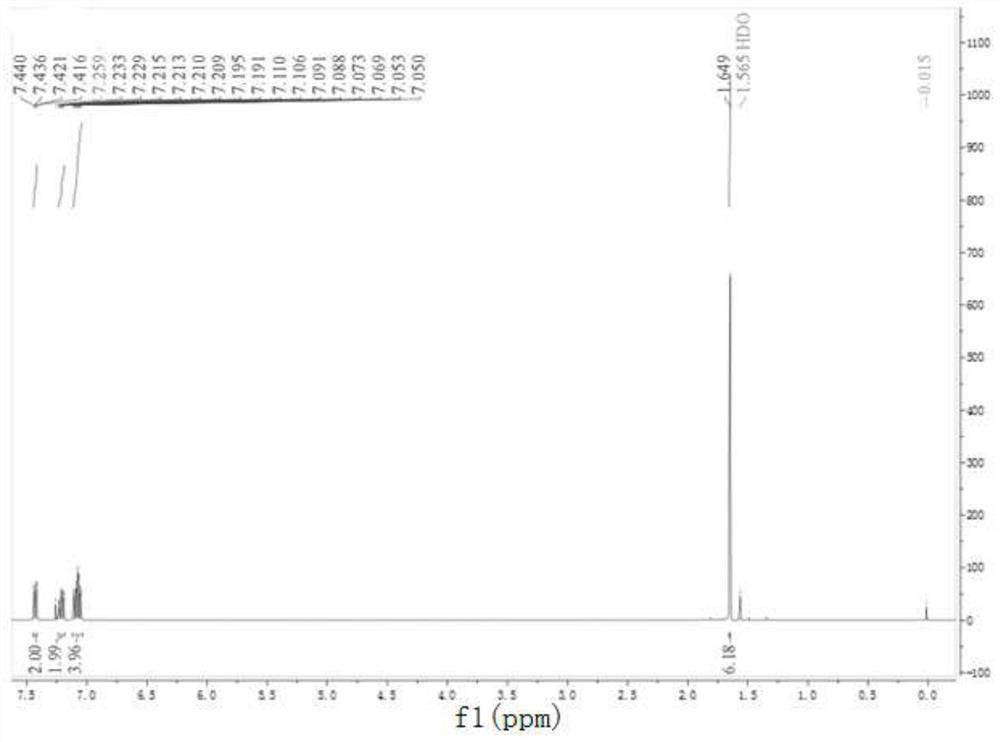
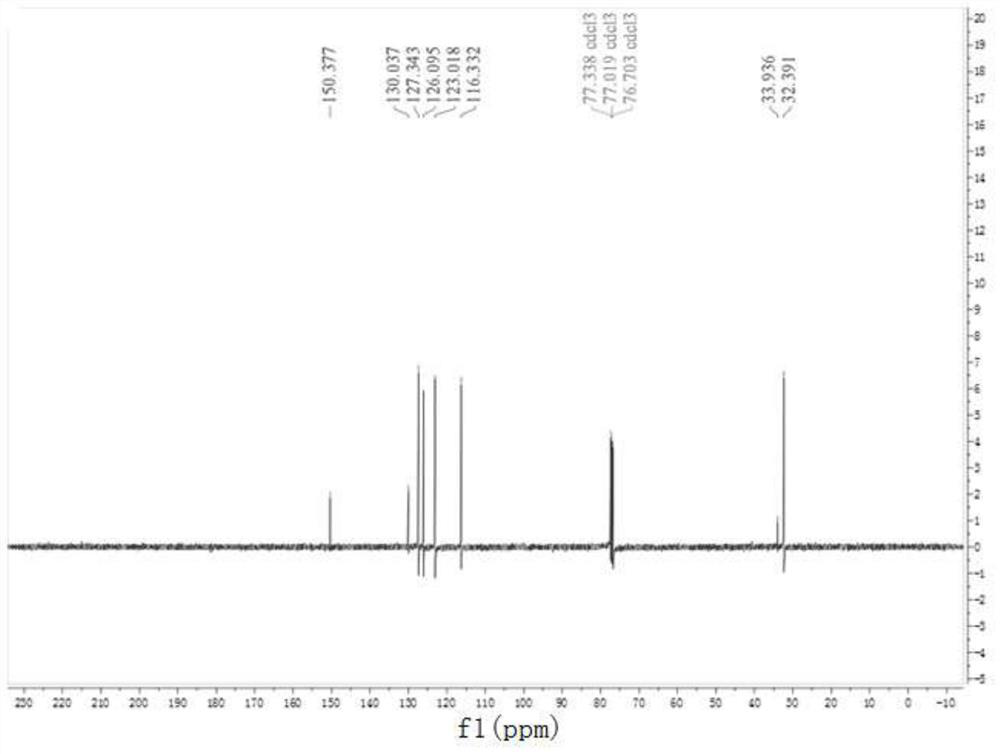
Preparation method of 9,9-dimethyl xanthene
A technology of dimethyl xanthene and diphenyl ether, applied in 9 fields, can solve the problems of low production efficiency, high production equipment requirements, and high equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Take a 500ml three-neck flask, add 150ml trifluoromethanesulfonic acid and 78g diphenyl ether in sequence, and slowly add 26g of acetone dropwise after the addition is completed. After the addition, raise the temperature to 120°C and keep it for 2 hours. After cooling down to room temperature, pour it into ice water. Adjust the pH to neutral with 20% sodium hydroxide. A large number of light yellow crystals precipitated after standing overnight, filtered, the filtrate was extracted with 500ml of dichloromethane and dried with anhydrous sodium sulfate, the organic layer was rotary evaporated to obtain 83g of crude product, the crude product was recrystallized with 200ml of dichloromethane / methanol mixed solvent 70.6 g of pure product were obtained. The yield was 75%.
Embodiment 2
[0024] Take a 2L four-neck flask, add 50g of acetone and 154g of diphenyl ether in sequence, fill it with argon for protection after the addition, then slowly add 92ml of concentrated sulfuric acid dropwise, after the dropwise temperature rise to 80°C, and keep it warm for 5h. After the reaction was completed, the temperature was lowered to room temperature, the reactants were poured into ice water, and the pH was adjusted to be neutral with 20% sodium hydroxide in mass percent. After standing overnight, it was extracted with 1 L of dichloromethane and dried with anhydrous sodium sulfate. The organic layer was rotary evaporated to obtain 150 g of crude product, which was recrystallized with 600 ml of dichloromethane / methanol mixed solvent to obtain 128 g of pure product. The yield was 70.7%.
Embodiment 3
[0026] Take a 2L four-neck bottle, add 254g methanesulfonic acid and 150g diphenyl ether in sequence, fill with argon after the addition is complete, then slowly add 50g of acetone dropwise, after the dropwise increase the temperature to 120°C, and keep it warm for 5 hours. After the reaction was completed, the temperature was lowered to room temperature, the reactant was poured into ice water, and the pH was adjusted to be neutral with 30% sodium hydroxide by mass percentage. After standing overnight, it was extracted with 1 L of dichloromethane and dried with anhydrous sodium sulfate. The organic layer was rotary evaporated to obtain 157 g of crude product, which was recrystallized with 600 ml of dichloromethane / methanol mixed solvent to obtain 135 g of pure product. The yield was 74.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com