Application of human amniotic epithelial cells in preparation of medicine for preventing or treating senile asthenia
A technology for senile debilitation and epithelial cells, which is applied in the direction of drug combinations, pharmaceutical formulas, and medical raw materials derived from mammals, etc., to achieve the effect of simple separation methods, improved living conditions, and no ethical problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The human amniotic epithelial cells used in this example are the P3 generation cells isolated and cultured from human amniotic membrane. Select 20 (10 male and 10 male) healthy mice with similar body weight at the age of 8-10 and no clinical symptoms after observation. Two groups, the experimental group and the control group, with 10 mice in each group (5 female mice and 5 male mice), were weighed and recorded. The experimental group was given therapeutic agents (ingredients: containing 1×10 6 Human amniotic epithelial cells (0.5mL normal saline), the control group was given placebo (ingredient: 0.5mL normal saline), tail vein injection administration. After 2 months, observe the clinical symptoms (observation in separate cages, the observation contents include whether death, dying, activity status, appearance and coat, trauma, feces, etc.), and weigh them. Then the mice were dissected, and the main organs (heart, liver, spleen, lung, kidney, testis (male), ovary (fema...
Embodiment 2
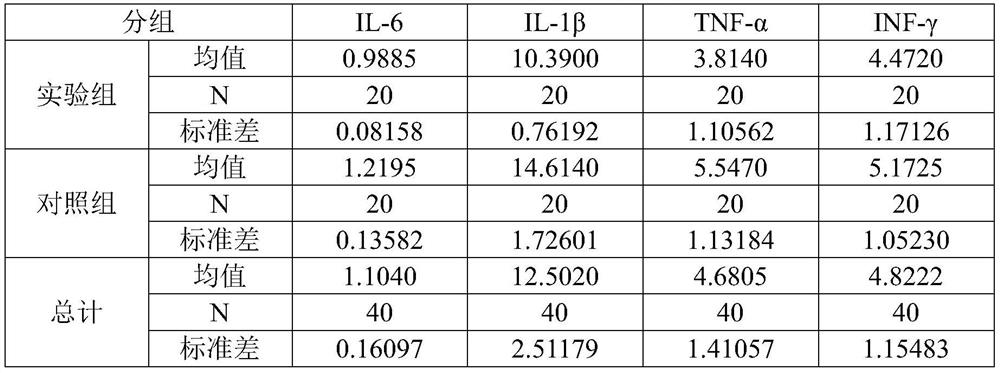
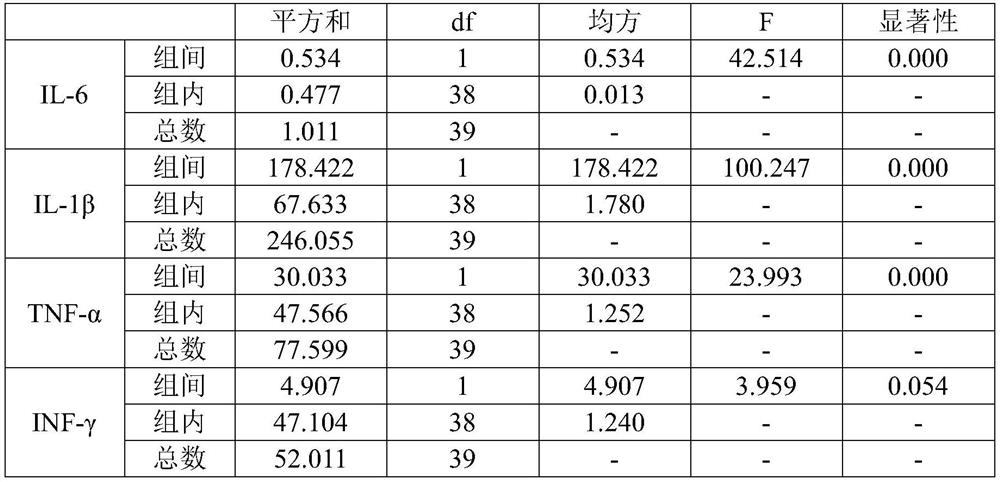
[0034] The human amniotic epithelial cells used in this example are the P3 generation cells isolated and cultured from human amniotic membrane, and 40 8-week-old IL-10-deficient mice (female mice) were purchased and raised under SPF (Specific Pathogen Free, no specific pathogen) conditions. 20 mice, 20 male mice), were evenly divided into two groups, an experimental group and a control group, with 20 mice in each group (10 female mice, 10 male mice). Before the experiment, blood was collected from the submandibular vein of each mouse, and the levels of inflammatory factors IL-6, IL-1β, TNF-α and IFN-γ in the serum were determined by MSD ultra-sensitive electrochemiluminescence method, and then the experimental group was given therapeutic agents (components : Including 1×10 6 Human amniotic epithelial cells (0.5mL normal saline), the control group was given placebo (ingredient: 0.5mL normal saline), tail vein injection administration. After 8 weeks, blood was collected from ea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com