Preparation method of cyclic gamma-polyglutamic acid modified hydrogel loaded with antibacterial drug
A technology of polyglutamic acid and antibacterial drugs, applied in the fields of biotechnology and pharmacy, can solve problems such as unreported, and achieve the effect of good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the preparation of cyclo-γ-PGA modified PNIPAM hydrogel
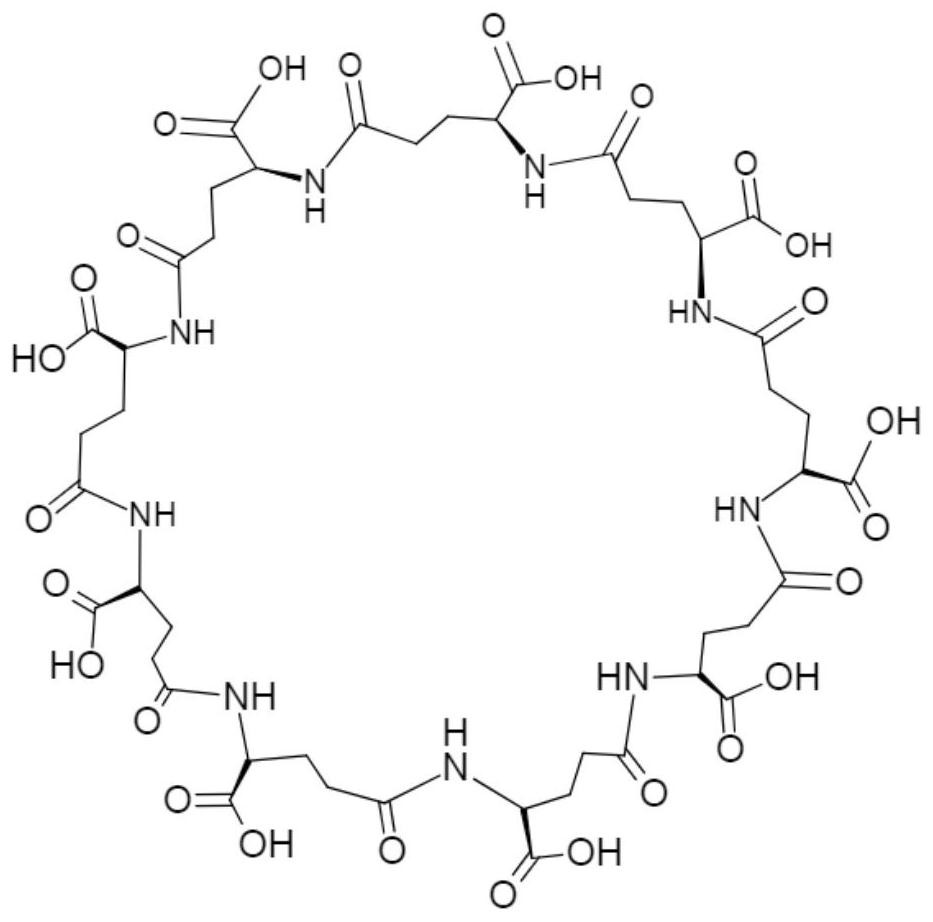
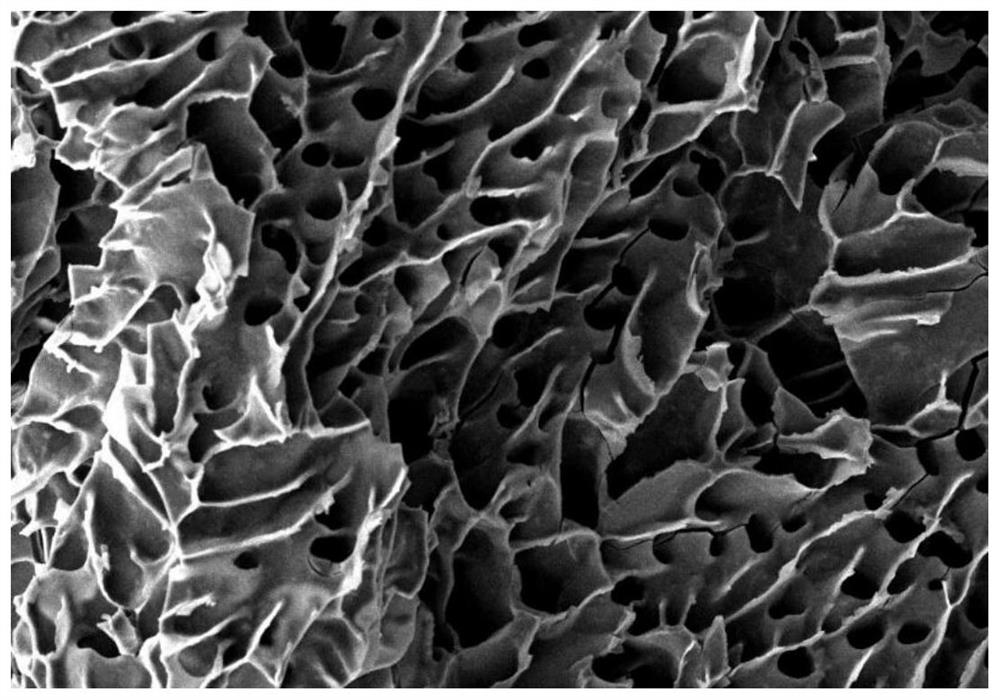
[0037] cyclo-γ-PGA ( figure 1 ) Modified PNIPAM hydrogel: Mix the two substances according to PNIPAM / cyclo-γ-PGA in order of 100:0, 75:25, 50:50, and 25:75, and the total concentration of the solution is 20% (w / v) , mixed well at room temperature for 3 hours, debubbled overnight at 4°C, and gelled in a water bath at 37°C. It can be observed that the dehydration of PNIPAM hydrogels has been effectively improved when PNIPAM / cyclo-γ-PGA is 75:25 or 50:50 ( figure 2 ). Under the scanning electron microscope, when PNIPAM / cyclo-γ-PGA was 75:25, the hydrogel showed a typical porous structure ( image 3 ).
Embodiment 2
[0038] Embodiment 2: Preparation of cyclo-γ-PGA modified PNIPAM hydrogel loaded with erythromycin
[0039] Mix erythromycin at a final concentration of 0.5 mg / ml, 150 mg / mL PNIPAM and 50 mg / mL cyclo-γ-PGA, mix thoroughly at room temperature for 3 hours, remove air bubbles overnight at 4°C, and gel in a water bath at 37°C. The water retention rate of the obtained hydrogel is 90%, which has a good water retention effect; the bacteriostasis rate is 75%, and has a good antibacterial effect.
Embodiment 3
[0040] Embodiment 3: the preparation of the cyclo-γ-PGA modified PNIPAM hydrogel of loaded neomycin
[0041] Mix neomycin, 150 mg / mL PNIPAM, and 50 mg / mL cyclo-γ-PGA at a final concentration of 1 mg / ml, mix thoroughly at room temperature for 3 hours, remove air bubbles overnight at 4°C, and gel in a water bath at 37°C. The water retention rate of the obtained hydrogel is 90%, which has a good water retention effect; the bacteriostasis rate is 80%, and has a good antibacterial effect.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com