Azacyclopropene compound, and preparation method and application thereof
A technology of compounds and nitrogen heterocycles, applied in the field of molecular probes in chemical biology, can solve the problem of selective modification research but few
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0087] Compound preparation
[0088] In addition to standard methods known in the literature or exemplified in experimental procedures, the compounds of the invention can be prepared using the methods shown in the following synthetic schemes. Combined with the following synthesis scheme, the compounds and synthesis methods described in the present invention can be better understood. The synthetic schemes describe methods that can be used to prepare the compounds described in the present invention, and the methods described are illustrative scheme descriptions for illustrative purposes only and are not intended to limit the scope of the invention.
[0089]
[0090] Synthetic Scheme 1
[0091]
[0092] Synthetic Scheme 2
[0093] Compound application
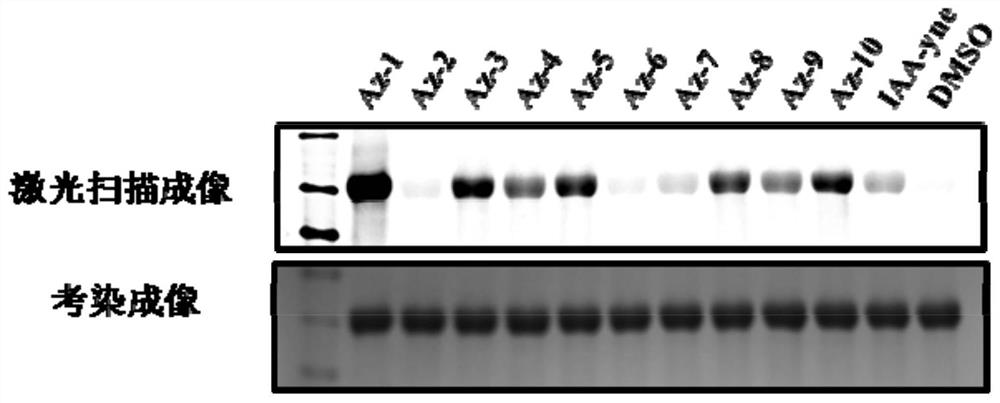
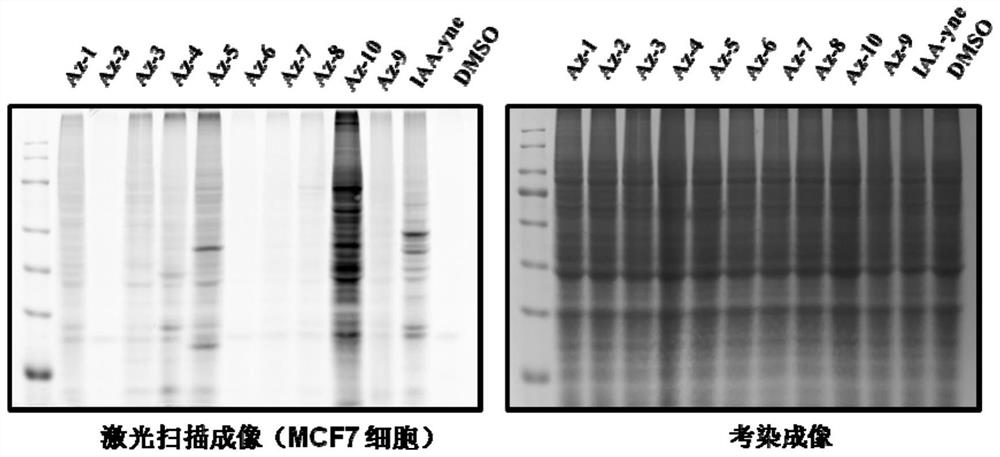
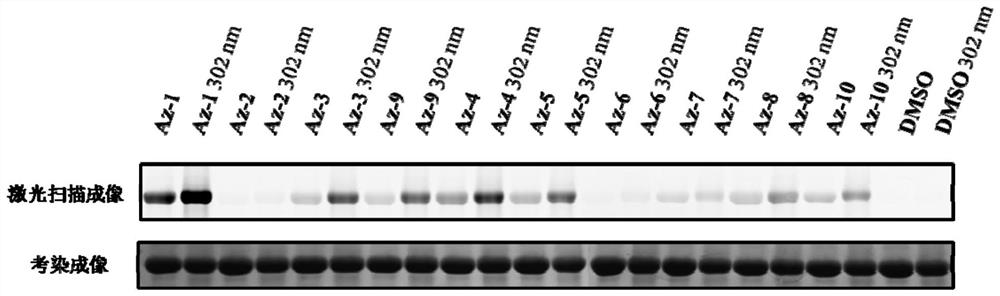
[0094] The present invention also provides the application of the above-mentioned aziridine compound. The compound of formula I provided by the present invention has the effect of labeling the amino acid residues of prot...
Embodiment 1
[0104]
[0105] In the first step, compound 1 (8 mL, 48.2 mmol) was dissolved in dry THF (5 mL), LDA (6.58 mL, 53.1 mmol) was added under argon protection at -15°C, and reacted at 0°C for 30 minutes. Then 3-bromopropyne (4.24 mL, 53.1 mmol) was added and reacted at room temperature for 12 h. with saturated NH 4 The Cl solution was quenched and extracted with ethyl acetate. The organic layers were combined and dried over anhydrous sodium sulfate. After filtration, the solvent was evaporated under reduced pressure, and compound 2 (8.32 g, 88%) was obtained by silica gel column chromatography. 1 H NMR (400MHz, CDCl 3 )δ3.36(s,2H),2.85–2.73(m,2H),2.50–2.42(m,2H),1.96–1.94(m,1H),1.46(s,12H).
[0106] In the second step, compound 2 (3.0g, 15.2mmol) was added to NH 2 OH·HCl (1.17g, 16.72mmol) in pyridine (10mL) was reacted at 50°C for 24h, the solvent was removed under reduced pressure, diluted with water and extracted with ethyl acetate. The organic layers were combined and ...
Embodiment 2
[0109]
[0110] In the first step, compound 4 (8mL, 48.2mmol) was dissolved in dry THF (5mL), sodium hydride (1.95g, 48.7mmol) was added under the protection of argon at 0°C, and reacted at 0°C for 30 minutes. Then 3-bromopropyne (4.24 mL, 53.1 mmol) was added and reacted at room temperature for 12 h. with saturated NH 4 The Cl solution was quenched and extracted with ethyl acetate. The organic layers were combined and dried over anhydrous sodium sulfate. After filtration, the solvent was evaporated under reduced pressure, and compound 5 (7.57 g, 80%) was obtained by silica gel column chromatography. 1 H NMR (400MHz, CDCl 3 )δ3.56(t, J=7.5Hz, 1H), 2.63(dt, J=7.6, 2.2Hz, 2H), 2.26(s, 3H), 1.96(t, J=2.7Hz, 1H), 1.44( s,9H).
[0111] The synthesis of compound 6 refers to the synthesis of compound 3 in Example 1.
[0112] Synthesis of Compound Az-2 Reference Example 1 Synthesis of Compound Az-1, yield 40%. 1 H NMR (400MHz, CDCl 3 )δ3.14(dd, J=18.3,2.7Hz,1H),2.71(dd,J=18....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com