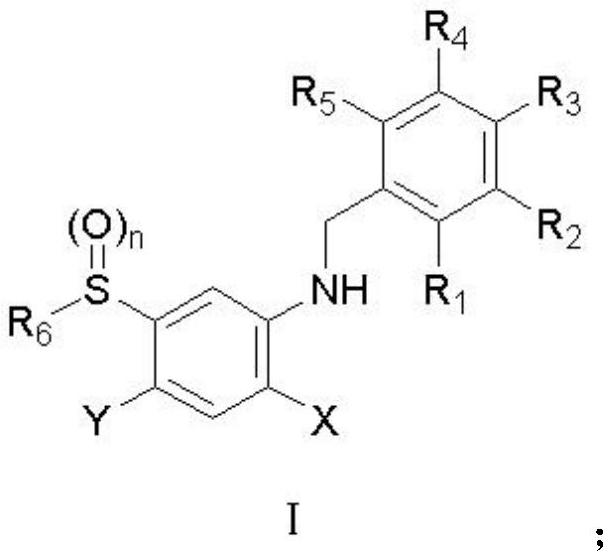
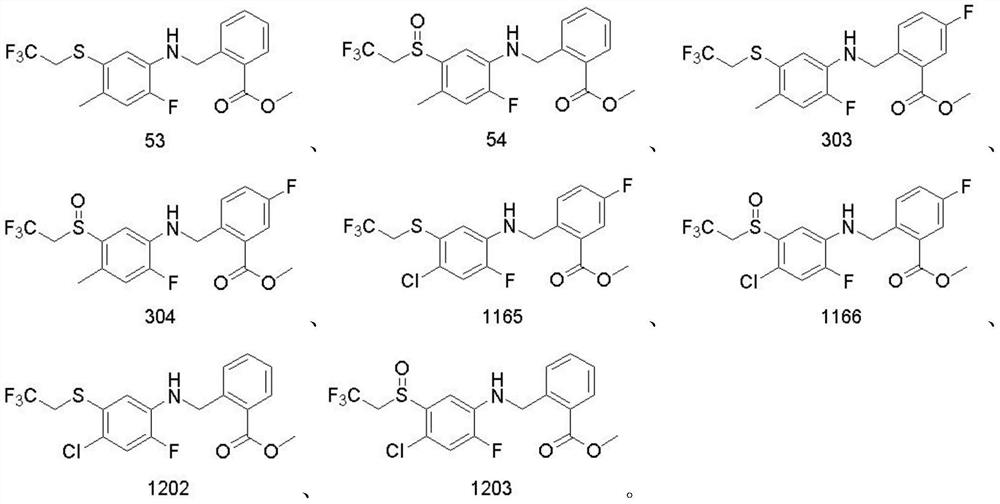
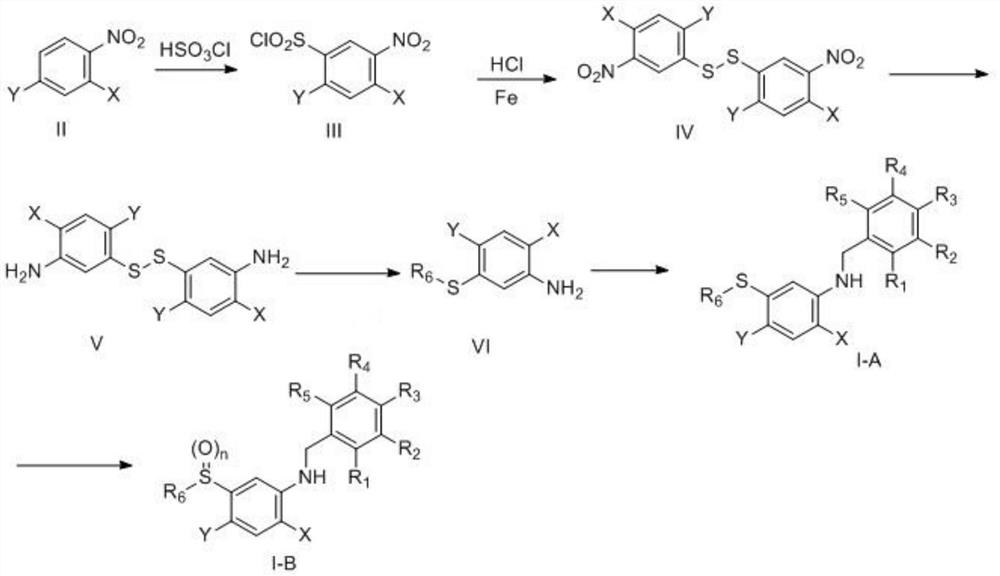
Aryl sulfide containing benzylamine structure and synthesis method and application thereof
A technology containing benzylamine and sulfide, applied in the field of pesticides, can solve the problems of low killing activity, poor control effect of spider mites, unsatisfactory acaricidal activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] Preparation of N-(4-chloro-3-fluorobenzyl)-2-fluoro-4-methyl-5-((2,2,2-trifluoroethyl)thio)aniline (Compound 249)
[0127] Step 1: Preparation of 4-fluoro-2-methyl-5-nitrobenzenesulfonyl chloride
[0128]
[0129] At room temperature, chlorosulfonic acid (34.95g, 30mmol) was added to a 250mL round bottom flask, 2-fluoro-4-methylnitrobenzene (15.5g, 10mmol) was slowly added to the reaction flask in 4 batches, and the reaction The liquid has a significant exotherm. After the addition, the reaction flask is transferred to 60 degrees Celsius and heated, and the reaction is basically completed after 2 hours. The reaction solution was added to 500 mL of ice-water mixture, 400 mL of dichloromethane was added, and the liquid was separated after rapid extraction. The organic phase was again added with 300 mL of water, and the organic phase was evaporated to dryness after extraction and separation. Yellow solid 16.5g (yield 64.45%).
[0130] Step 2: Preparation of 1,2-bis(4-fluoro-2-m...
Embodiment 2
[0153] Preparation of 2-fluoro-N-(3-fluorobenzyl)-4-methyl-5-((2,2,2-trifluoroethyl)thio)aniline (compound 229)
[0154] Step 1: Preparation of 4-fluoro-2-methyl-5-nitrothiophenol
[0155] Place 4-fluoro-2-methyl-5-nitrobenzenesulfonyl chloride (0.15mol, 38.0g) in a 250ml single-necked flask, add 80ml of hydroiodic acid, the reaction system will turn black, stir and react at room temperature for 1 hour, then 80ml of saturated aqueous sodium sulfite solution was slowly added, and a yellow powdery solid appeared in the reaction system. The solid was filtered under reduced pressure and washed with water and dried to obtain 28.0 g of light yellow powder with a yield of 100%.
[0156] Step 2: Preparation of 4-fluoro-2-methyl-5-aminothiophenol
[0157] Dissolve 4-fluoro-2-methyl-5-nitrothiophenol (0.15mol, 28.0g) in absolute ethanol (280mL), add 10% palladium on carbon (1.0g, 50% water content) , Hydrogen replaces the gas in the reaction flask three times, and the reaction solution is sti...
Embodiment 3
[0166] Preparation of 2-fluoro-N-(3-methoxybenzyl)-4-methyl-5-((2,2,2-trifluoroethyl)thio)aniline (Compound 239)
[0167] Step 1: Preparation of N-(2-fluoro-4-methylphenyl)acetamide
[0168]
[0169] Dissolve 2-fluoro-4methyl aniline (125g, 1mol) in dichloromethane (1L), add triethylamine (111g, 1.1mol), cool to internal temperature 0 degrees Celsius in an ice salt bath, slowly add acetic anhydride dropwise (102g, 1mol), after the addition, the reaction solution was transferred to room temperature and the reaction was continued for 3 hours. 2L of water was added to the reaction solution, and the liquid was extracted. The organic phase was dried by adding anhydrous sodium sulfate and evaporated to dryness to obtain 162g white solid , The yield is 97.0%.
[0170] Step 2: Preparation of 5-acetamido-4-fluoro-2-methylbenzenesulfonyl chloride
[0171]
[0172] Add N-(2-fluoro-4-methylphenyl)acetamide (145g, 868mmol) into a 1L round bottom flask, set up an exhaust gas absorption device, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com