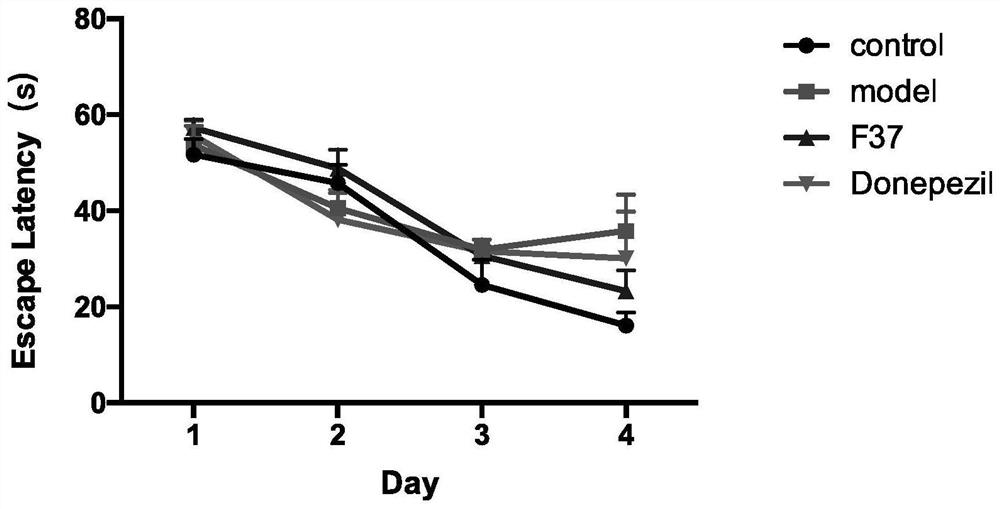
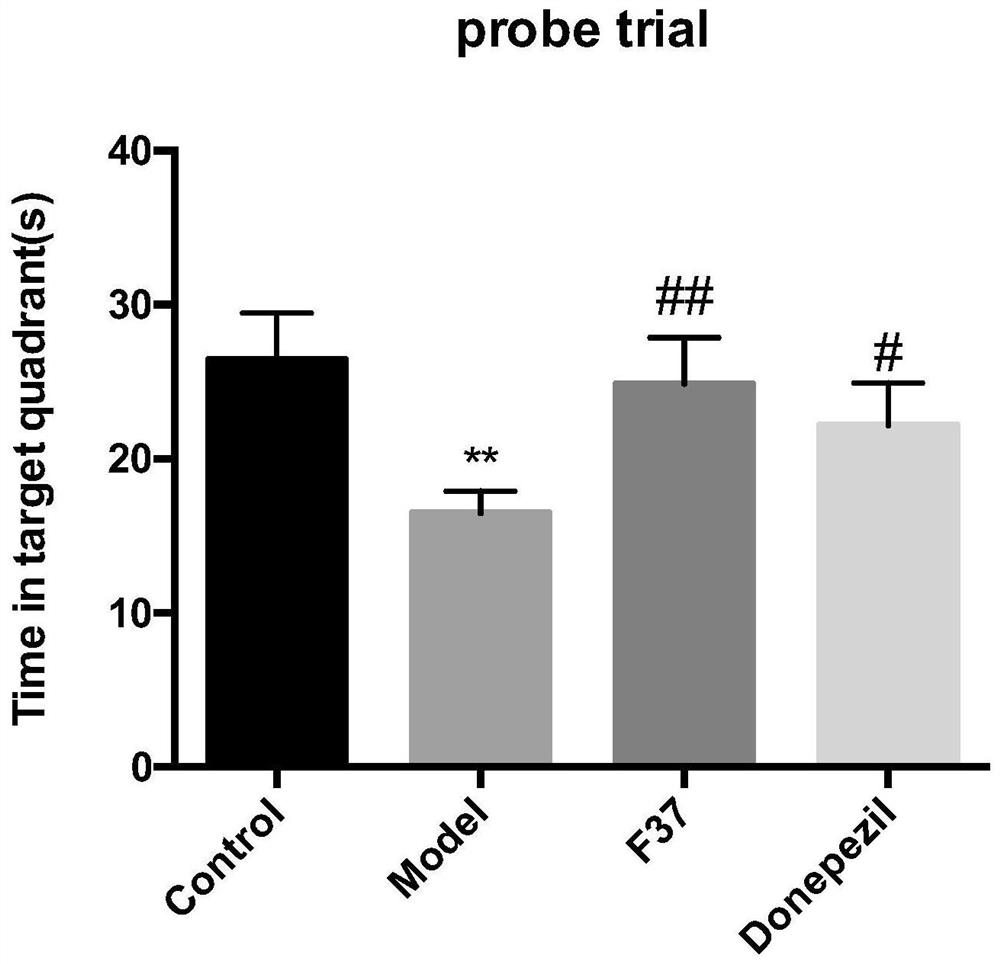
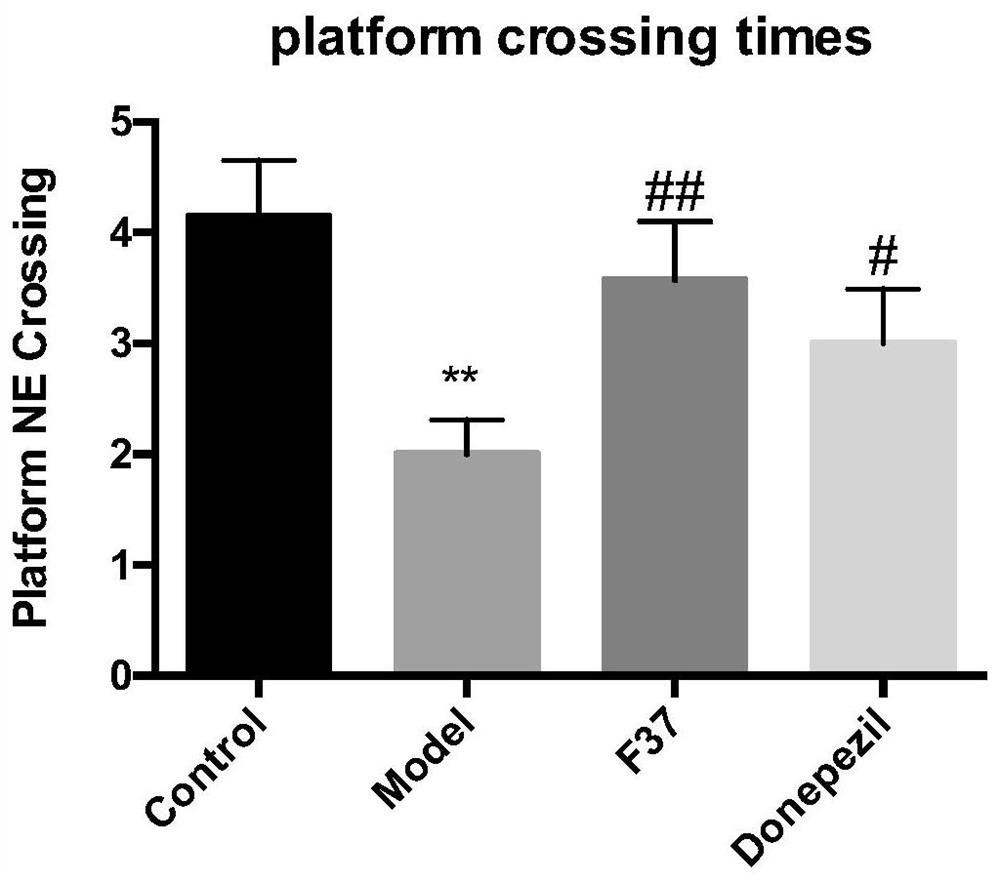
Steroid sapogenin derivative as well as preparation method and application thereof
A compound, unsubstituted technology, applied in the field of medicinal chemistry, which can solve the problems of reduced mitochondrial redox activity, damage, and high content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0131] The synthesis of compound F is as follows:
[0132]
[0133] Synthesis of Intermediate D1:
[0134] Dissolve diosgenin (2mmol), aluminum isopropoxide (3mmol) and cyclohexanone (3mmol) in 20mL toluene, react at 95°C for 4h, use after the reaction is complete, spin evaporate toluene to dryness, dichloromethane / water extraction, The organic phases were combined, dried over anhydrous sodium sulfate, concentrated under reduced pressure and subjected to column chromatography (ethyl acetate / petroleum ether=1:15) to obtain compound D1 (80%).
[0135] Synthesis of Intermediate D2:
[0136] Dissolve compound D1 (1.5mmol) in 15mL of dioxane, add trimethylchlorosilane at low temperature, add 2,3-dichloro-5,6-dicyano-p-benzoquinone (3mmol) after 15min to react for 12h, and react After complete use, the solvent was evaporated to dryness, extracted with dichloromethane / water, the organic phases were combined, dried over anhydrous sodium sulfate, concentrated under reduced pressur...
Embodiment 2
[0140] The preparation of compound F1-F11 is shown in the following reaction formula
[0141]
[0142] in, express
[0143] Preparation of Compounds F1~F11
[0144] Compound F (396mg, 1mmol), the corresponding carboxylic acid (1.2mmol), EDCl (1.2mmol), DMAP (0.05mmol) were dissolved in 20mL of dichloromethane under the protection of argon, and reacted at room temperature for 3h. After the reaction was completed, quenched with 30 mL of water, extracted with dichloromethane (3*30 mL), combined the organic phases, dried over anhydrous sodium sulfate, concentrated under reduced pressure and then subjected to column chromatography (ethyl acetate / petroleum ether=1: 20) Obtain compounds F1-F11 (75%-85%).
[0145] Compound F1, white solid. 1 H NMR (400MHz, CDCl 3 )δ7.27 (1H, d, J = 8.3Hz), 6.83 (1H, dd, J = 8.4Hz, 2.5Hz), 6.79 (1H, d, J = 2.3Hz), 4.46 (1H, m), 3.49 (1H,dd,J=9.9Hz,5.0Hz),3.39(1H,t,J=10.9Hz),2.88(2H,m),2.28(3H,s),1.00(3H,d,J=6.8Hz ),0.81(3H,s),0.80(3H,d,J=6...
Embodiment 3
[0157] Preparation of Compounds F12~F15
[0158]
[0159] in, express
[0160] Preparation of Compounds F12~F15
[0161] Compound F (396mg, 1mmol), the corresponding sulfonyl chloride (1.2mmol), TEA (1.2mmol) were dissolved in 20mL of dichloromethane under the protection of argon, and reacted at room temperature for 3h. After the reaction was completed, quenched with 30 mL of water, extracted with dichloromethane (3*30 mL), combined the organic phases, dried over anhydrous sodium sulfate, concentrated under reduced pressure and then subjected to column chromatography (ethyl acetate / petroleum ether=1: 20) Obtain compounds F12-F15 (80%-90%).
[0162] Compound F12, white solid. 1 H NMR (400MHz, CDCl 3 )δ7.23 (1H, d, J = 8.5Hz), 7.01 (1H, d, J = 8.5Hz), 6.98 (1H, s), 4.46 (1H, dd, J = 13.9Hz, 7.8Hz), 4.46 (1H,dd,J=13.9Hz,7.8Hz),3.49(1H,dd,J=9.8Hz,4.7Hz),3.39(1H,t,J=10.9Hz),2.89(2H,m),1.00( 3H,d,J=6.7Hz),0.82(3H,s),0.80(3H,d,J=6.4Hz).C 28 h 40 o 5 S MS(EI):m / z 489.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com