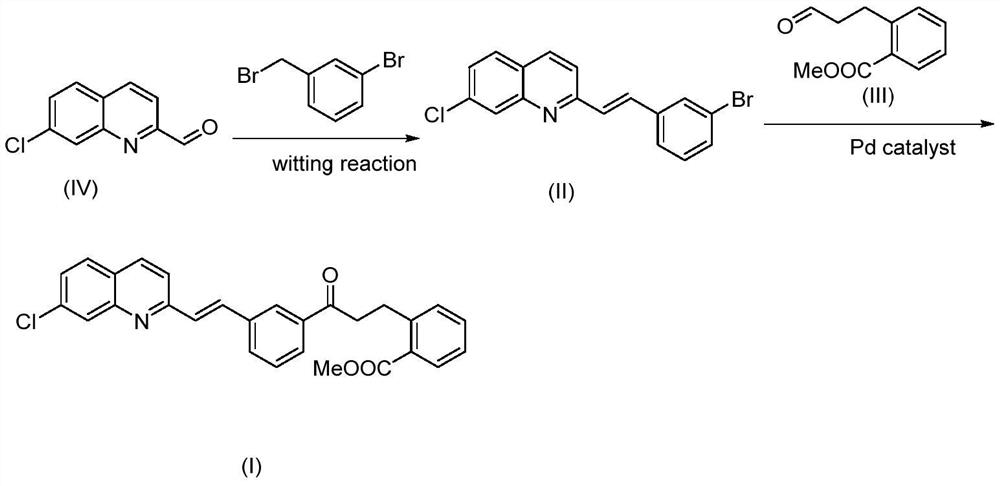
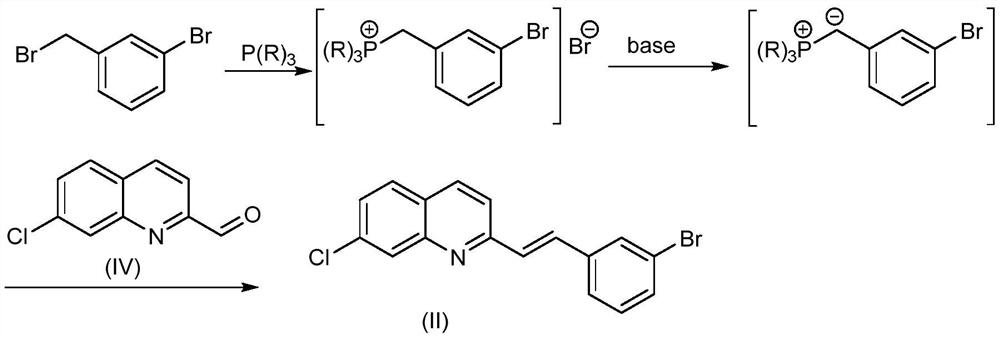
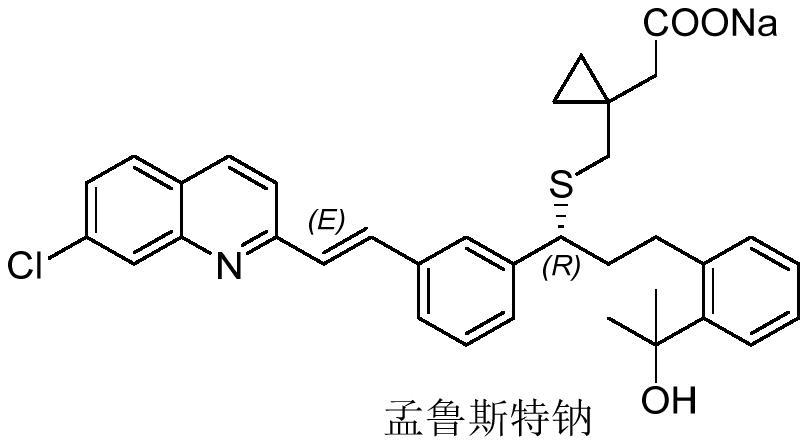
Synthesis method of montelukast sodium intermediate
A technology of montelukast sodium and its synthetic method, which is applied in the field of drug synthesis, can solve the problems of being unsuitable for industrial production, harsh reaction conditions, and long production cycle, and achieve the effects of low cost, short process route, and easy operation and production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: the synthesis of formula (II) compound
[0036]
[0037] 3-Bromobenzyl bromide (97.8g, 391.4mmol) and triphenylphosphine (102.7g, 391.4mmol) were dissolved in DMF (500mL), heated to 120°C, stirred for 2h, cooled to 0°C and stirred for 30min, added Sodium methoxide (42.3g, 782mmol) was stirred for 30min, then the compound of formula (IV) (50g, 260.9mmol) was slowly added, the temperature was gradually raised to room temperature, and the mixture was reacted for 1h. TLC detected that the reaction of the raw materials was complete. Pour the reaction solution into 1L of 1mol / L dilute hydrochloric acid, add 1L of ethyl acetate for extraction, wash the organic phase with saturated sodium bicarbonate solution and brine successively, collect the organic phase, dry, decolorize, and concentrate under reduced pressure. The concentrated solution was added into ice water, stirred for 30 min, the solid gradually precipitated out, filtered, rinsed with aqueous sodium ...
Embodiment 2
[0038] Embodiment 2: the synthesis of formula (II) compound
[0039]
[0040] 3-Bromobenzyl bromide (65.2g, 260.9mmol) and triphenylphosphine (68.4g, 260.9mmol) were dissolved in THF (500mL), heated to 60°C, stirred for 12h, cooled to 0°C and stirred for 30min, added Potassium tert-butoxide (35.1g, 313.1mmol) was stirred for 30min, then the compound of formula (IV) (50g, 260.9mmol) was slowly added, the temperature was gradually raised to room temperature, and the mixture was reacted for 2h. The reaction of the raw materials was detected by TLC. Pour the reaction solution into 1L of 1mol / L dilute hydrochloric acid, add 1L of ethyl acetate for extraction, wash the organic phase with saturated sodium bicarbonate solution and brine successively, collect the organic phase, dry, decolorize, and concentrate under reduced pressure. The concentrated solution was added into ice water, stirred for 30 min, the solid precipitated out, filtered, washed with aqueous sodium bicarbonate ...
Embodiment 3
[0041] Embodiment 3: the synthesis of formula (II) compound
[0042]
[0043] 3-Bromobenzyl bromide (71.7g, 287.0mmol) and triethylphosphine (33.9g, 287.0mmol) were dissolved in toluene (500mL), heated to 100°C, stirred for 6h, cooled to 0°C and stirred for 30min, added Sodium hydride (17.2g, 430mmol) was stirred for 30min, then the compound of formula (IV) (50g, 260.9mmol) was slowly added, and the temperature was gradually raised to room temperature, and the mixture was reacted for 2h. TLC detected that the reaction of the raw materials was complete. Pour the reaction solution into 1L of 1mol / L dilute hydrochloric acid, add 1L of ethyl acetate for extraction, wash the organic phase with saturated sodium bicarbonate solution and brine successively, collect the organic phase, dry, decolorize, and concentrate under reduced pressure. The concentrated solution was added into ice water, stirred for 30 min, the solid precipitated out, filtered, washed with aqueous sodium bicar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com