Bromophenol-pyrazoline compound and synthesis method and application thereof
A synthesis method and compound technology, applied in the field of compounds, can solve problems such as repeated infection, difficulty in complete immunity, unsatisfactory treatment effect, etc., and achieve broad application prospects and high-efficiency inhibition of activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
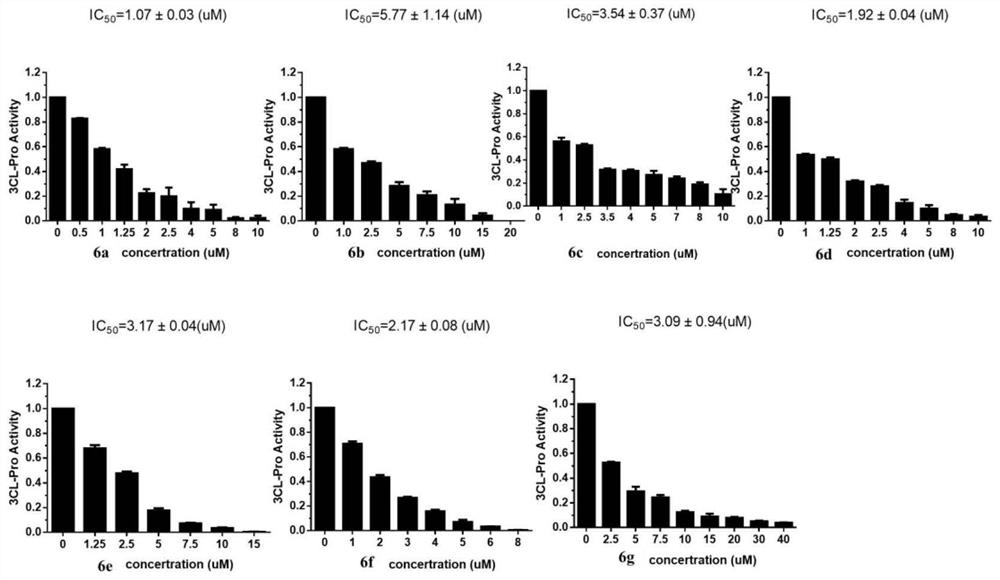
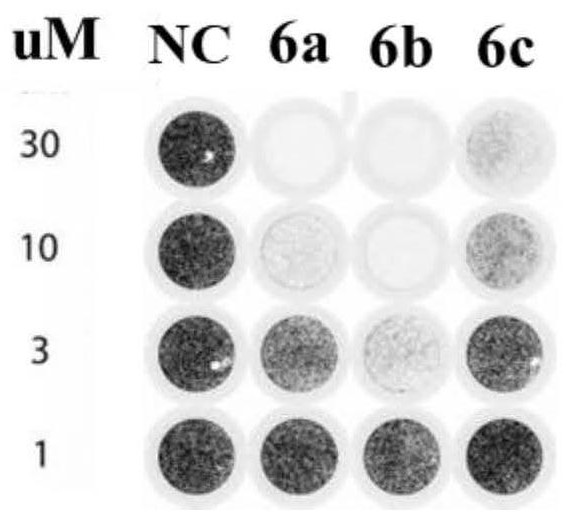
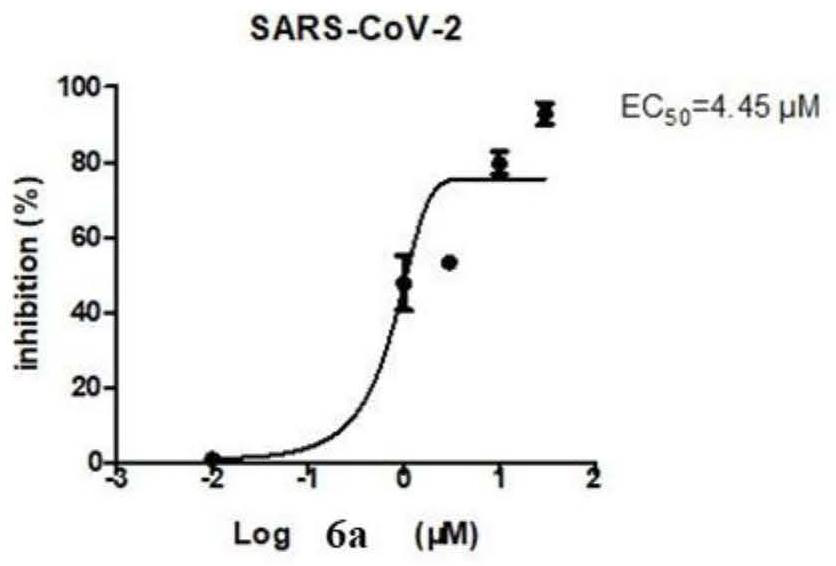
[0026] Example 1: 3,4-dibromo-5-(3-(4-(4-ethoxyphenoxy)phenyl)-4,5-dihydro-1H-pyrazol-5yl)-1 , Synthesis and characterization of 2-dihydroxybenzene (6a)
[0027] (1) Synthetic route and specific steps
[0028]
[0029] Take 15.2g of vanillin (0.1mol, 1) and add it into 100mL of dichloromethane, and stir evenly. Under stirring in an ice bath, a solution of 5.6 mL of bromine (16.0 g, 0.1 mol) in 10 mL of methanol was added dropwise. React at room temperature for 2 hours, then cool to 0°C. 50 mL of ice water was added dropwise, and a precipitate precipitated out, filtered, washed with ice water, and dried to obtain 21 g of white solid 5-bromo-vanillin (2a, yield 92%, mp 160-162°C).
[0030] Add 21g of 5-bromo-vanillin (2a, 0.09mol) into 100mL of acetic acid, and add dropwise a solution of 10.26mL (0.2mol) of bromine in 15mL of acetic acid under normal temperature stirring. Add 0.1 g of reduced iron powder, and reflux for 2 hours. Cool to room temperature, filter the preci...
Embodiment 2
[0038] Example 2: 5-(2,3-dibromo-4,5-dihydroxyphenyl)-3-(4-(4-ethoxyphenoxy)phenyl)-4,5-dihydro- Synthesis and Characterization of 1H-1-Formaldehyde Pyrazole (6b)
[0039] (1) Synthetic route and specific steps
[0040]
[0041] Weigh 267 mg (0.5 mmol) of compound (5a), dissolve it in a mixed solution of 3 mL ethanol and 1 mL formic acid, and add 5 drops of hydrazine hydrate (concentration 85%) under stirring. Heating to reflux for 2h, cooling, and standing overnight. A white solid precipitated, after ultrasonic vibration, filtered, washed with water and ethanol to obtain the final product 5-(2,3-dibromo-4,5-dihydroxyphenyl)-3-(4-(4-ethoxy (phenoxy)phenyl)-4,5-dihydro-1H-pyrazole-1-carbaldehyde (6b), 176 mg (61.1% yield).
[0042] (2) Compound characterization
[0043] 1 H NMR (500MHz, DMSO-d6) δ8.94(s, 1H), 7.75(d, J=8.3Hz, 2H), 7.04(d, J=8.9Hz, 2H), 6.97(dd, J=13.6, 8.8Hz, 4H), 6.53(s, 1H), 5.63(dd, J=11.4, 3.8Hz, 1H), 4.03–4.00(m, 2H), 3.91(dd, J=17.8, 11.8Hz, 1H),...
Embodiment 3
[0045] Example 3: 5-(2,3-dibromo-4,5-dihydroxyphenyl)-3-(4-(4-ethoxyphenoxy)phenyl)-4,5-dihydro- Synthesis and Characterization of 1H-1-Acetylpyrazole (6c)
[0046] (1) Synthetic route and specific steps
[0047]
[0048] Weigh 267 mg (0.5 mmol) of compound (5a), dissolve it in 4 mL of acetic acid solution, and add 5 drops of hydrazine hydrate (concentration 85%) under stirring. Heating to reflux for 2h, cooling, and standing overnight. A white solid precipitated, after ultrasonic vibration, filtered, washed with water and ethanol to obtain the final product 5-(2,3-dibromo-4,5-dihydroxyphenyl)-3-(4-(4-ethoxy (phenoxy)phenyl)-4,5-dihydro-1H-1-acetylpyrazole (6c), 121 mg (yield 41.1%).
[0049] (2) Compound characterization
[0050] 1 H NMR (500MHz, DMSO-d6) δ9.97(s, 1H), 9.54(s, 1H), 7.73(d, J=8.8Hz, 2H), 7.01(d, J=9.0Hz, 2H), 6.98 –6.91(m,4H),6.46(s,1H),5.61(d,J=7.8Hz,1H),4.05–3.98(m,2H),3.89–3.82(m,1H),2.95(dd,J =17.9,4.0Hz,1H),2.31(s,3H),1.31(t,J=7.0Hz,3H).
[005...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com