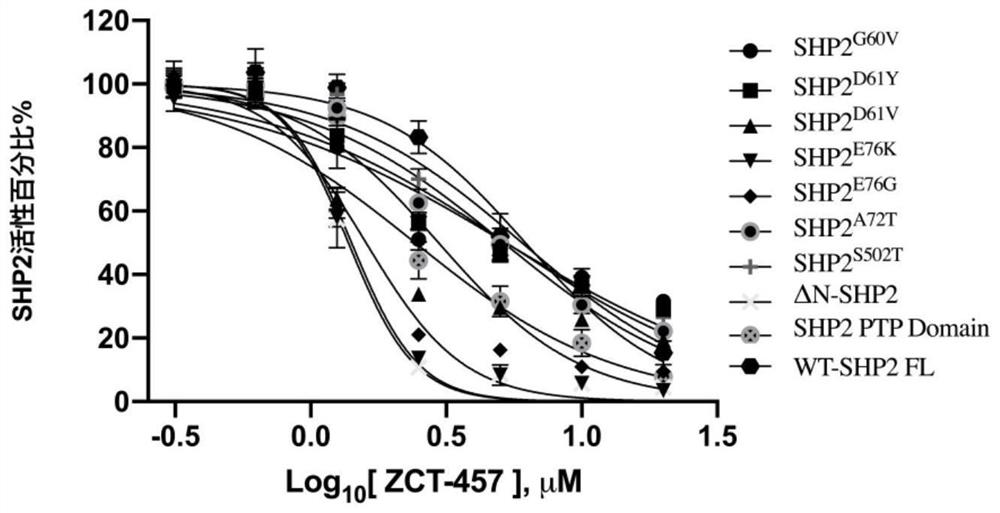
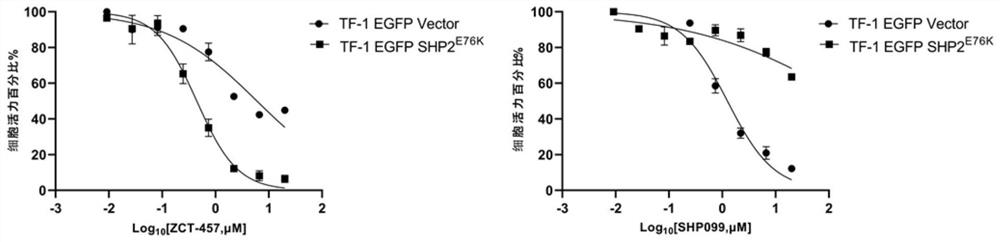
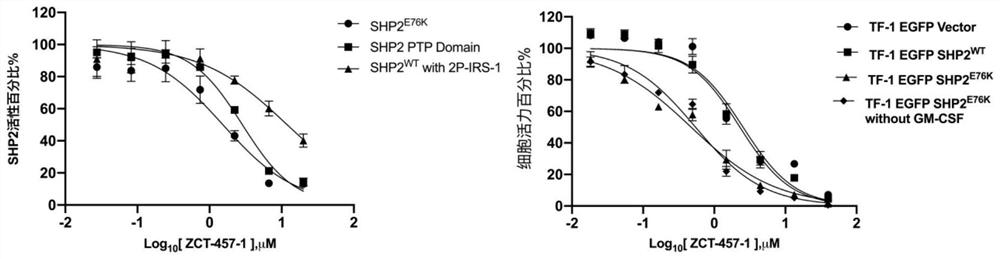
Oxygen-containing five-membered heterocyclic compound, synthesis method, pharmaceutical composition and application
A five-membered heterocycle and compound technology, applied in the field of medicine and its preparation and application, can solve problems that cannot meet the needs of clinical drug development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0125] Example 1 Synthesis of oxygen-containing five-membered heterocyclic compounds
[0126] Preparation of important intermediates:
[0127]
[0128] Reagents and conditions: a) acetonitrile, 120°C; b) iron powder, ammonium chloride, ethanol and water at 90°C; c) diethyl oxalate, 150°C.
[0129] Under ice bath conditions, 2-propylamine (3.54 g, 0.06 mol) was slowly added dropwise to a solution of 4-fluoro-3-nitrobenzonitrile (5 g, 0.03 mol) in acetonitrile (40 mL), stirred for 5 min, and then placed in the solution. The reaction was refluxed in an oil bath at 120 °C for 1.5 h. After monitoring the completion of the reaction, dichloromethane (200 mL) and hydrochloric acid (200 mL, 1 mol / L) were added for extraction. The organic phase was collected, dried over anhydrous sodium sulfate, and concentrated to obtain compound I-1. (6.17 g, 100% yield). 1H NMR (400MHz, CDCl 3 )δ8.52(d,J=2.0Hz,1H),8.35(s,1H),7.59(dd,J=9.1,1.8Hz,1H),6.92(d,J=9.1Hz,1H),3.88( m,1H),1.37(d,J=6.4 H...
Embodiment 2
[0175] Example 2 Synthesis of oxygen-containing five-membered heterocyclic compounds
[0176]
[0177] Reagents and conditions: a) N,N'-carbonyldiimidazole, dichloromethane; b) hydrazine hydrate, methanol;
[0178] A solution of 2-furancarboxylic acid (2 g, 0.018 mol) in dichloromethane (20 mL) was activated with N,N'-carbonyldiimidazole (3.2 g, 0.02 mol), and after monitoring complete activation, methyl 3-aminobenzoate was added The ester (2.72g, 0.018mol) was placed at room temperature and reacted overnight. After monitoring the completion of the reaction, a large amount of dichloromethane was added, washed 3 times with saturated aqueous sodium bicarbonate solution, and then washed 3 times with hydrochloric acid (1mol / L), and vacuumed After drying, the product was recrystallized from ethyl acetate to obtain a white solid product II-1 (3.8 g, yield 81.5%). 1 H NMR (400MHz, DMSO-d 6 )δ10.41(s, 1H), 8.43(t, J=1.9Hz, 1H), 8.04(m, 1H), 7.96(m, 1H), 7.69(dt, J=7.9, 1.3Hz, 1H)...
Embodiment 3
[0210] Example 3 Synthesis of oxygen-containing five-membered heterocyclic compounds
[0211] Preparation of important intermediates:
[0212]
[0213] Under ice bath conditions, isopropylamine (2.69 g, 45.56 mol) was slowly added dropwise to a solution of 2-fluoro-5-bromonitrobenzene (5 g, 22.73 mol) in acetonitrile (40 mL), stirred for 5 min and then placed at 120 °C The reaction was refluxed in an oil bath for 1.5 h, and after monitoring the completion of the reaction, dichloromethane (200 mL) and hydrochloric acid (200 mL, 1 mol / L) were added for extraction, the organic phase was collected, dried over anhydrous sodium sulfate, and concentrated to obtain compound III-1 (5.26 g, 90% yield). 1 H NMR (400MHz, DMSO-d 6 )δ8.16(d,J=2.5Hz,1H),7.89(d,J=7.7Hz,1H),7.65(dd,J=9.3,2.5Hz,1H),7.09(d,J=9.3Hz, 1H),3.93(dq,J=13.1,6.5Hz,1H),1.26(d,J=6.3Hz,6H).MS(ESI):m / z calcd.For C 9 H 12 BrN 2 O 2 [M+H] +259.0, found 259.0
[0214] will contain compound III-1 (5 g, 0.02 mol) and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com