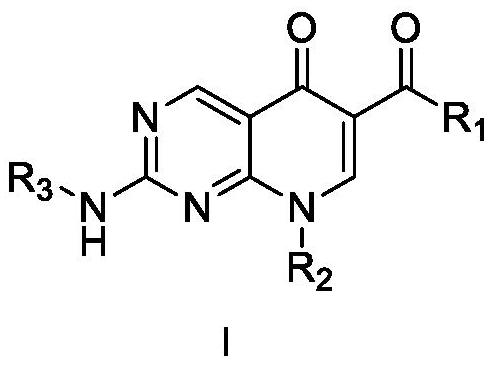
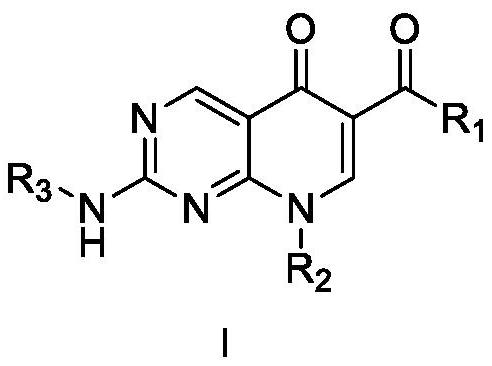
Diarylpyrimido pyridone derivative, preparation method and application thereof
A technology for diarylpyrimidine-pyridone and its derivatives, which is applied in the field of derivatives and their preparation, diarylpyrimidine-pyridone derivatives and their preparation, and can solve the problems of poor oral availability and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
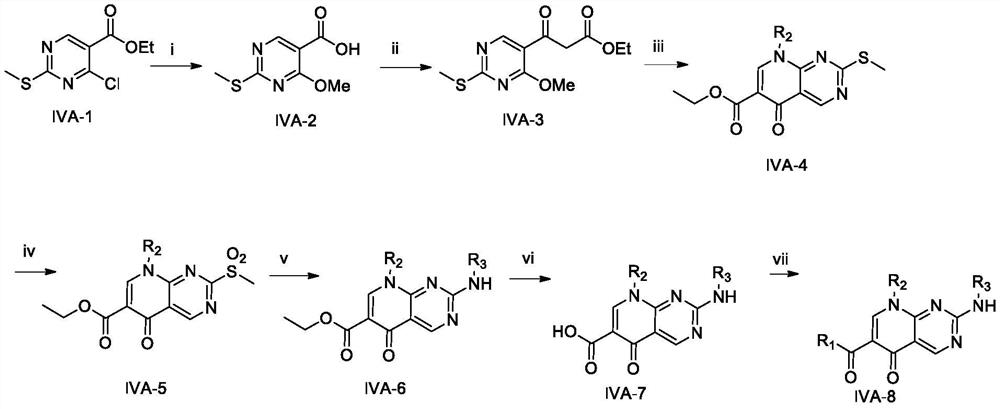
[0083] Example 1: Preparation of 4-methoxy-2-(methylthio)-5-pyrimidinecarboxylic acid (IVA-2)
[0084] Weigh ethyl 4-chloro-2-methylthio-5-pyrimidinecarboxylate (2 g, 8.6 mmol) into a 100 mL eggplant-shaped flask, and add 30 mL of MeOH and 10 mL of 2N NaOH solution in sequence. After stirring at room temperature for 3 hours, most of the methanol was evaporated to dryness. The resulting solution was acidified with 2N dilute hydrochloric acid solution to pH 4-5, filtered to obtain a pure white solid, and dried in vacuo overnight. It was directly submitted to the next step without further purification.
Embodiment 2
[0085] Example 2: Preparation of 3-(4-methoxy-2-methylsulfanylpyrimidin-5-yl)-3-oxopropionic acid ethyl ester (IVA-3)
[0086] 4-Methoxy-2-(methylthio)-5-pyrimidinecarboxylic acid (1.6g, 8mmol) was dispersed in dichloromethane (30mL) and cooled in an ice bath, then added dropwise to One drop of DMF and oxalyl chloride (2.3 mL, 25.8 mmol). Stirring was continued at room temperature for 4 hours after the addition was complete. The reaction solvent was evaporated to give a pure white solid, which was used in the next step without further purification.
[0087] Under nitrogen protection, add methylmagnesium bromide ether solution (3M, 14.3mL, 42.9mmol) dropwise to a solution of monoethyl malonate (2.3mL, 21.4mmol) in anhydrous THF (15mL) in an ice bath . After the dropwise reaction was continued for 20 minutes, a THF (30 mL) solution of the above acid chloride was added dropwise to the reaction system. After stirring at room temperature for 3 hours, the reaction solution was p...
Embodiment 3
[0088] Embodiment 3: IVA-4 preparation general method
[0089] Potassium carbonate method: the above-mentioned 3-(4-methoxy-2-methylsulfanylpyrimidin-5-yl)-3-oxopropionic acid ethyl ester (1.0g, 3.7mmol), acetic anhydride (1.0 mL, 10mmol) and triethyl orthoformate (1.0mL, 6mmol) were heated to reflux at 130°C for 4h, then concentrated under reduced pressure to obtain a brown oil. Add THF 15mL to the above oil, add aniline or mesitylene or 4-amino-3,5-dimethylbenzonitrile (4.1mmol) under stirring condition, room temperature overnight. The reaction solution was evaporated to dryness, 15 mL of anhydrous DMF and potassium carbonate (0.6 g, 4.1 mmol) were added, and heated at 105° C. for 8 h under nitrogen protection. After the reaction, most of the DMF was evaporated to dryness under reduced pressure, 50 mL of water was added, extracted with dichloromethane (3*20 mL), the organic phases were combined, washed with saturated brine and dried over anhydrous sodium sulfate. The organ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com