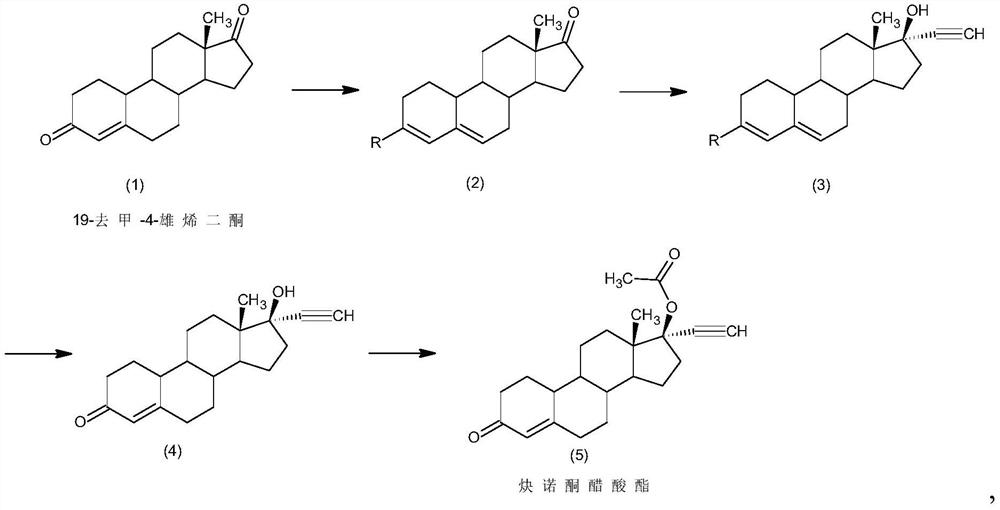
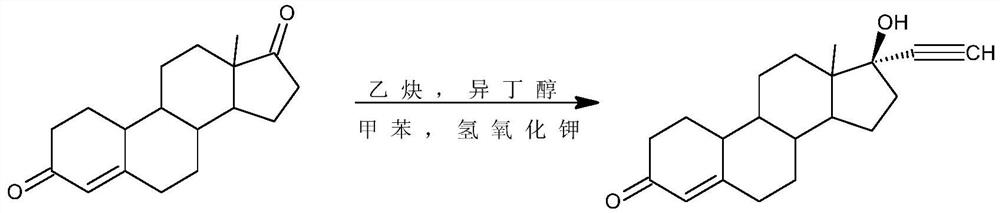
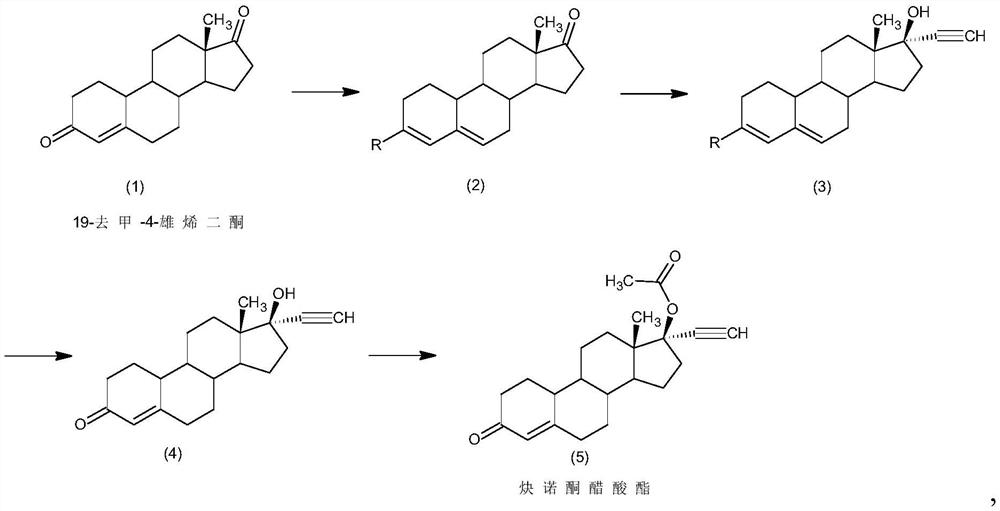
Preparation method of norethindrone acetate
A technology of norethisterone acetate and a synthesis method is applied in the field of drug preparation, can solve the problems of large 3-position acetylenic impurities, is unsuitable for industrial production, difficult to purify and the like, achieves mild reaction, is suitable for industrial production and operation low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A kind of preparation method of norethindrone acetate of embodiment 1 comprises the steps:
[0030] 1) Protection reaction: Add 50g of 19-nor-4-androstenedione (1) into 50ml of methanol, add 250ml of trimethyl orthoformate, 5g of pyridine hydrochloride, control the temperature at 30°C and stir the reaction. After the reaction, add 5ml of triethylamine, cooled to 5°C, filtered and dried to obtain 51.5g of intermediate 2;
[0031] 2) Alkynylation reaction: 51.5g of intermediate (2) obtained in step 1) was added to 750ml of acetone, 100g of potassium tert-butoxide was added, acetylene was introduced, and the reaction was stirred at 0°C. Sulfuric acid aqueous solution neutralized, concentrated, added water for water analysis, and filtered to obtain 53g of intermediate 3.
[0032] 3) Hydrolysis reaction: 53g of intermediate (3) obtained in step 2) was added to 750ml of tetrahydrofuran, and 250ml of 5% hydrochloric acid aqueous solution was added, and the reaction was stirre...
Embodiment 2
[0034] A kind of preparation method of norethindrone acetate of embodiment 2 comprises the steps:
[0035] 1) Protection reaction: Add 50g of 19-nor-4-androstenedione (1) into 500ml of ethanol, add 50ml of triethyl orthoformate, 0.5g of pyridinium hydrobromide, control the temperature at 20°C and stir the reaction. , add 0.5ml triethylamine, cool down to -5°C, filter and dry to obtain 53.0g intermediate 2;
[0036] 2) Alkynylation reaction: 53.0g of intermediate (2) obtained in step 1) is added to 300ml of tetrahydrofuran, 27g of potassium isobutoxide is added, acetylene is passed through, and the reaction is stirred at 20°C under control. After the reaction is completed, use 30% Neutralize with acetic acid aqueous solution, concentrate, add water for water analysis, and filter to obtain 54 g of intermediate 3.
[0037] 3) Hydrolysis reaction: 54g of intermediate (3) obtained in step 2) was added to 1500ml of acetone, 110ml of 10% hydrochloric acid aqueous solution was added,...
Embodiment 3
[0039] A kind of preparation method of norethindrone acetate of embodiment 3 comprises the steps:
[0040] 1) Protection reaction: Add 50g of 19-nor-4-androstenedione (1) into 1000ml of pyrrolidine, add 150ml of triethyl orthoformate, 10g of p-toluenesulfonic acid, and control the temperature at 60°C to stir the reaction. After the reaction, Add 10ml of pyridine, cool down to -10°C, filter and dry to obtain 55.0g of intermediate 2;
[0041]2) Alkynylation reaction: 55.0g of intermediate (2) obtained in step 1) is added in 1650ml of toluene, 275g of potassium ethylate is added, acetylene is passed through, and the reaction is stirred at 50° C. Neutralize, concentrate, add water for water analysis, and filter to obtain 57g of intermediate 3.
[0042] 3) Hydrolysis reaction: 57g of intermediate (3) obtained in step 2) was added to 300ml of methyl tetrahydrofuran, and 29ml of 30% sulfuric acid aqueous solution was added to control the stirring reaction at 0°C. After the reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com