Thermoplastic acrylic resin and method for producing same, and resin composition
An acrylic resin and thermoplastic technology, applied in the direction of single-component synthetic polymer rayon, textiles and papermaking, fiber chemical characteristics, etc., can solve the problems of high solvent recovery cost and high drainage load
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0089] The present invention will be described in more detail below by way of examples. In addition, this invention is not limited to the following Example.
[0090] First, various measurement methods and evaluation methods will be described.
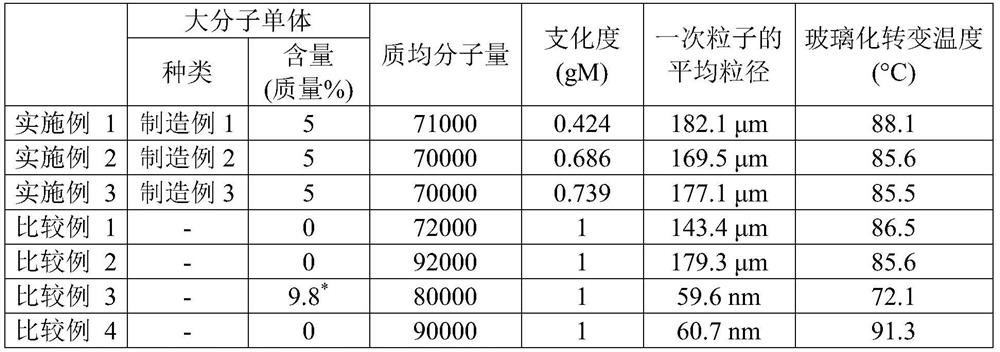
[0091] (1) Mass-average molecular weight and number-average molecular weight were measured and calculated by the GPC method using gel permeation chromatography ("HLC-8320GPC" manufactured by Tosoh Corporation).
[0092] (2) The average particle diameter is the particle diameter D50 at which the cumulative volume percentage is 50% by volume in the volume particle size distribution measured using a laser diffraction / scattering particle size distribution analyzer "Partica LA-950V2" manufactured by Horiba Corporation.
[0093] (3) The glass transition temperature of the acrylic resin is a value obtained by using a differential scanning calorimeter "DSC-6100" manufactured by Seiko Instruments Inc. to increase the temperature from -80°C to ...
manufacture example 1
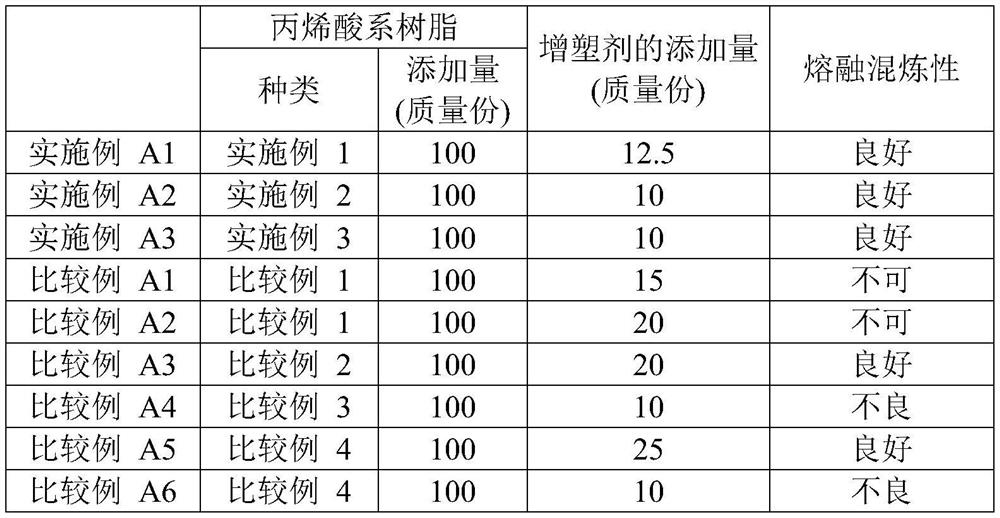
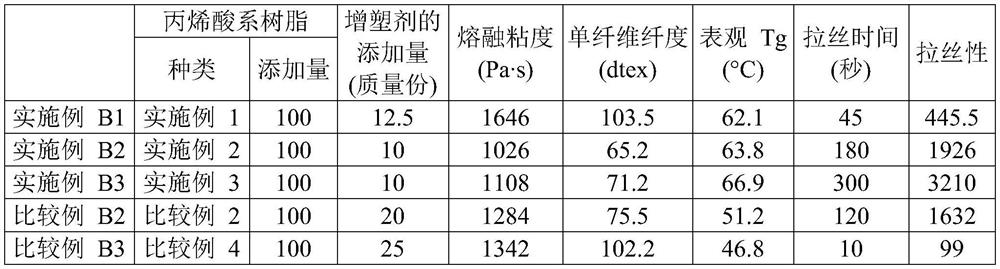
[0105] 554 g of CuBr was charged in a 2 L separable flask equipped with a reflux tube and a stirrer, and the inside of the reaction container was replaced with nitrogen. Next, 73.8 mL of acetonitrile was added, the separable flask was placed in a 70° C. oil bath, and the contents were stirred for 30 minutes. Then, 132 g of n-butyl acrylate, 7.2 mL of methyl 2-bromopropionate, and 4.69 mL of pentamethyldiethylenetriamine were added to a separable flask to start the reaction. While heating and stirring at 70° C., n-butyl acrylate (528 g) was continuously added dropwise over 90 minutes, and further, it was heated and stirred at 70° C. for 80 minutes. The reaction mixture was diluted with toluene, passed through an activated alumina column, and then the volatile components were distilled off under reduced pressure to obtain single-terminal Br group poly(n-butyl acrylate).
[0106] Charge 800 mL of methanol into the flask, and cool to 0°C. Potassium tert-butoxide (130 g) was adde...
manufacture example 2
[0109] Except for using 2-methoxyethyl acrylate instead of n-butyl acrylate, and changing the feed amount of 2-bromopropionate methyl to 14.4 mL, a single-ended acryloyl polyacrylate was obtained in the same manner as in Production Example 1. (2-methoxyethyl acrylate). The number average molecular weight of the obtained single-end acryloyl poly(2-methoxyethyl acrylate) macromonomer was 6000, and the molecular weight distribution (mass average molecular weight / number average molecular weight) was 1.24.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com