Method for synthesizing key intermediate of venetoclax
A synthesis method and intermediate technology, applied in the field of pharmaceutical synthesis, can solve the problems of harsh reaction conditions, high activity of Grignard reagents, and many impurities, and achieve the effects of simple operation, improved substitution reaction yield, high yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
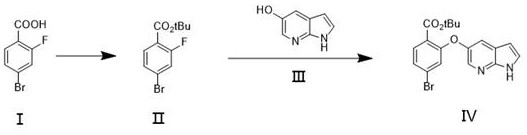
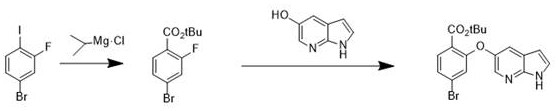
[0024] In the first step, add DMAP (0.367g, 3%) in 300mL THF solution, then add 4-bromo-2-fluorobenzoic acid (21.9g, 100mmol) and boc anhydride (26.16g, 120mmol) and stir, at 20 Reaction at ℃ for 1 hour, the solution after the esterification reaction was washed with 10% citric acid aqueous solution (200mL) and 10% sodium bicarbonate solution (200ml), dried over anhydrous sodium sulfate, and evaporated to dryness. The solution was tert-butyl 4-bromo-2-fluorobenzoate.
[0025] In the second step, 4-bromo-2-fluorobenzoic acid tert-butyl ester and 5-hydroxy-7-azaindole (14.74g, 110mmol) obtained from the first step reaction were dissolved in 200mL acetonitrile solution and 0.138g Potassium carbonate was reacted for 30 minutes, and the reaction temperature was 0°C. Then the solution after the reaction was quenched with water and filtered, and the filter cake was recrystallized and dried by adding ethyl acetate to obtain the key intermediate 4-bromo-2-[(1H-pyrrolo[2,3-b]pyridine of...
Embodiment 2
[0027] In the first step, add DMAP (9.16g, 50%) in 350mL THF solution, then add 4-bromo-2-fluorobenzoic acid (32.85g, 150mmol) and boc anhydride (87.2g, 400mmol) and stir, at 60 Reaction at ℃ for 1 hour, the solution after the esterification reaction was washed with 10% citric acid aqueous solution (200mL) and 10% sodium bicarbonate solution (200ml), dried over anhydrous sodium sulfate, and evaporated to dryness. The solution was tert-butyl 4-bromo-2-fluorobenzoate.
[0028] In the second step, 4-bromo-2-fluorobenzoic acid tert-butyl ester and 5-hydroxy-7-azaindole (14.74g, 110mmol) obtained from the first step reaction were dissolved in 200mL acetonitrile solution and 0.138g Potassium carbonate was reacted for 30 minutes, and the reaction temperature was 40°C. Then the solution after the reaction was quenched with water and filtered, and the filter cake was recrystallized and dried by adding ethyl acetate to obtain the key intermediate 4-bromo-2-[(1H-pyrrolo[2,3-b]pyridine o...
Embodiment 3
[0030] In the first step, add DMAP (9.16g, 50%) in 400mL THF solution, then add 4-bromo-2-fluorobenzoic acid (32.85g, 150mmol) and boc anhydride (163.5g, 750mmol) and stir, at 100 Reaction at ℃ for 1 hour, the solution after the esterification reaction was washed with 10% citric acid aqueous solution (200mL) and 10% sodium bicarbonate solution (200ml), dried over anhydrous sodium sulfate, and evaporated to dryness. The solution was tert-butyl 4-bromo-2-fluorobenzoate.
[0031] In the second step, 4-bromo-2-fluorobenzoic acid tert-butyl ester and 5-hydroxy-7-azaindole (14.74g, 110mmol) obtained from the first step reaction were dissolved in 200mL acetonitrile solution and 0.138g Potassium carbonate was reacted for 30 minutes, and the reaction temperature was 80°C. Then the solution after the reaction was quenched with water and filtered, and the filter cake was recrystallized and dried by adding ethyl acetate to obtain the key intermediate 4-bromo-2-[(1H-pyrrolo[2,3-b]pyridine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com