A kind of mesoporous metal organic framework and its preparation method and application
A metal-organic framework and mesoporous technology, which is applied in the direction of organic active ingredients, medical preparations of non-active ingredients, medical preparations containing active ingredients, etc., to achieve high drug loading, low systemic side effects, and high purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
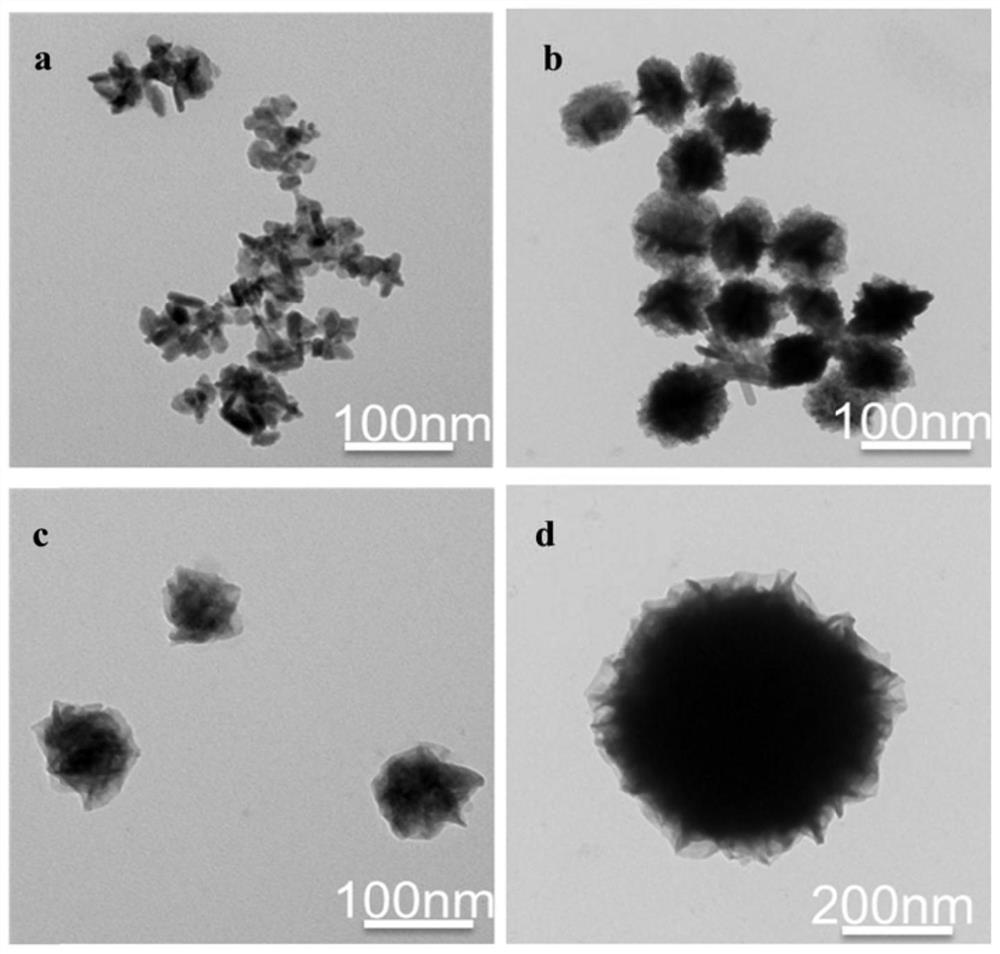
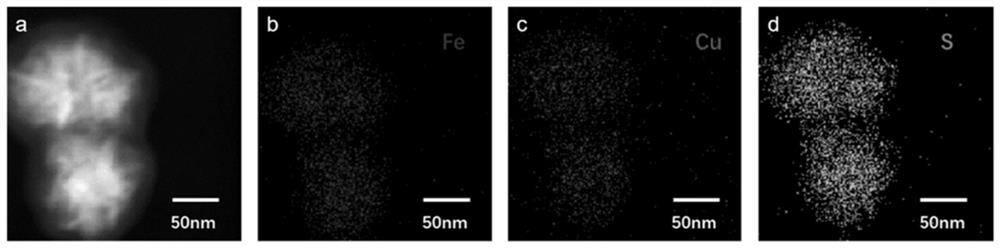
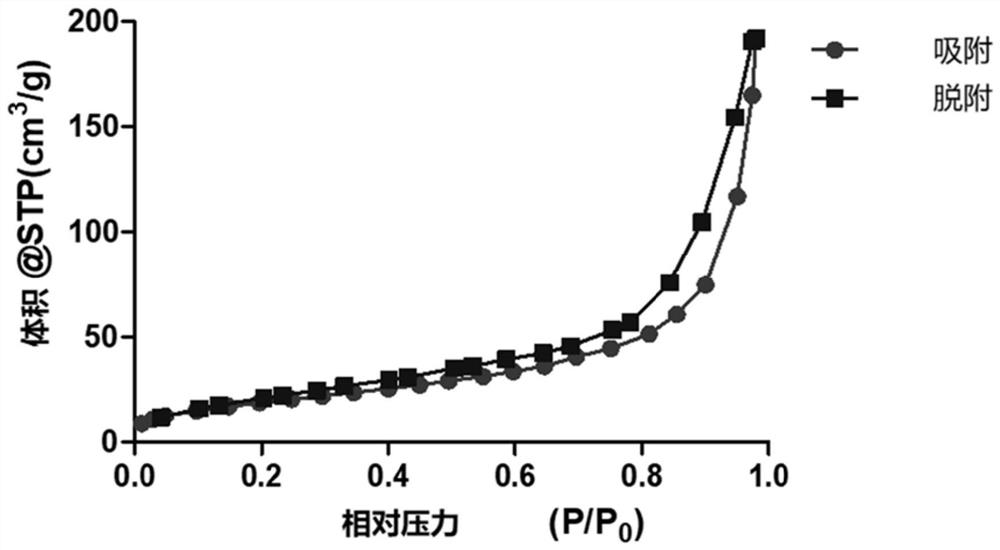
[0053] Embodiment 1: A kind of preparation method of mesoporous metal organic framework:
[0054] (1) get N,N-dimethylformamide and dehydrated alcohol, be mixed into the composite solution that volume ratio is 5:3;
[0055] (2) Take the composite solution obtained in step (1) to prepare FeCl with a mass concentration of 100 mg / mL respectively 2 Solution, containing CuCl with a mass concentration of 100 mg / mL 2 The solution and the dithioglycolic acid solution with a mass concentration of 50 mg / mL were dissolved by ultrasonic respectively;
[0056] (3) FeCl prepared by step (2) 2 Solution and CuCl 2 solution according to Fe 2+ and Cu 2+ The molar ratio of 1:1 was added to a 15mL centrifuge tube, then 0.1mL of dithioglycolic acid solution, 250mg of PVP and 100μL of TEA were added, and the compound solution in step (1) was used to make up to 15mL, and ultrasonically dissolved. , placed in an autoclave, and reacted at 150°C for 24h;
[0057] (4) Step (3) The reactant was al...
Embodiment 2
[0058] Embodiment 2: A kind of preparation method of mesoporous metal organic framework:
[0059] (1) get N,N-dimethylformamide and dehydrated alcohol, be mixed with the composite solution that volume ratio is 3:2;
[0060] (2) Take the composite solution obtained in step (1) to prepare FeCl with a mass concentration of 200 mg / mL respectively 2 Solution, containing CuCl with a mass concentration of 100 mg / mL 2 The solution and the selenocysteine solution with a mass concentration of 50 mg / mL were dissolved by ultrasonic respectively;
[0061] (3) FeCl prepared by step (2) 2 Solution and CuCl 2 solution according to Fe 2+ and Cu 2+ The molar ratio of 2:1 was added to a 15mL centrifuge tube, and then 0.1mL of selenocysteine solution, 300mg of PVP and 200μL of TEA were added, and the compound solution in step (1) was used to make up to 15mL. Dissolve by ultrasonic , placed in an autoclave, and reacted at 135°C for 24h;
[0062] (4) Step (3) The reactant was allowed to ...
Embodiment 3
[0063] Embodiment 3: A kind of preparation method of mesoporous metal organic framework:
[0064] (1) get N,N-dimethylformamide and dehydrated alcohol, be mixed into the composite solution that volume ratio is 5:3;
[0065] (2) Take the composite solution obtained in step (1) to prepare FeCl with a mass concentration of 150 mg / mL respectively 2 Solution, containing CuCl with a mass concentration of 200 mg / mL 2 The solution and the dithioglycolic acid solution with a mass concentration of 50 mg / mL were dissolved by ultrasonic respectively;
[0066] (3) FeCl prepared by step (2) 2 Solution and CuCl 2 solution according to Fe 2+ and Cu 2+ The molar ratio of 11:4 was added to a 15mL centrifuge tube, and then 0.1mL of dithioglycolic acid solution, 200mg of PVP and 80μL of TEA were added, and the composite solution in step (1) was used. , placed in an autoclave, and reacted at 150°C for 24h;
[0067] (4) Step (3) The reactant was allowed to stand for cooling at room temperatu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com