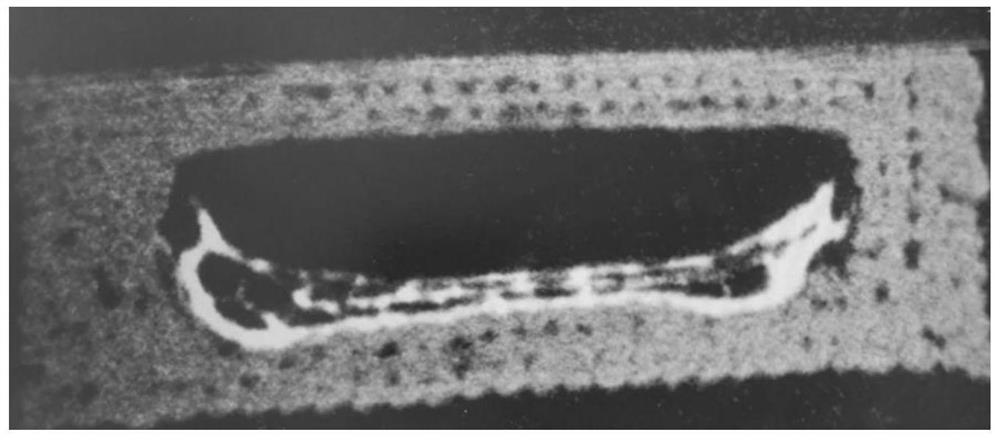
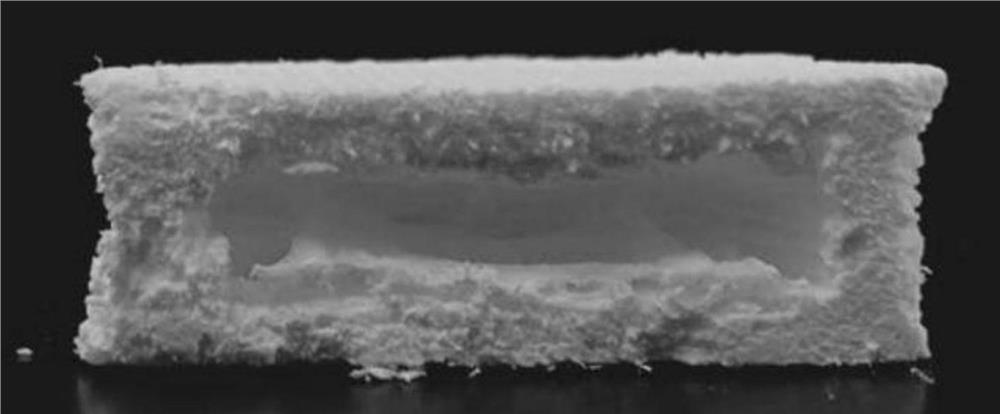
A kind of hollow gastric floating tablet of low-solubility drug prepared based on semi-solid 3D printing technology and preparation method thereof
A 3D printing and gastric floating tablet technology, which is applied in drug delivery, pharmaceutical formulations, antibacterial drugs, etc., can solve the problems of long drug release time and incomplete drug release, so as to prolong the residence time, reduce the number of drugs taken, and improve The effect of treatment compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
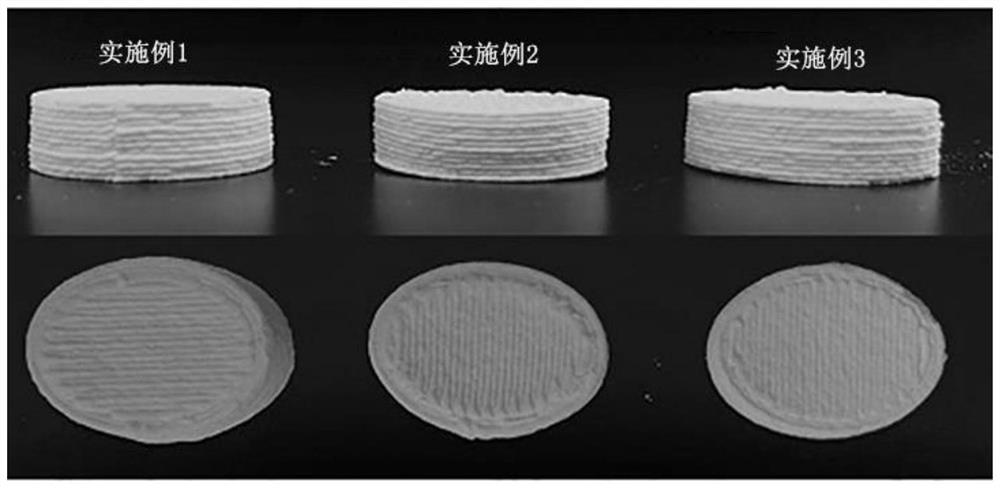
Embodiment 1
[0064] A preparation method of a hollow clarithromycin gastric floating tablet prepared based on a semi-solid 3D printing technology: the raw and auxiliary materials are respectively passed through a 90-mesh sieve;
[0065] 1: Take the release modifier (Poloxamer P407 0.10g), the binder (Povidone K30 0.70g), and the water-erodible polymer skeleton material (Hypromellose (K100 MCR) 0.90g), mix Evenly, add 15 mL of 95% ethanol, stir evenly, clarithromycin micropowder 5.4 g, and stir until solidified. Crush the solidified product, slowly add 3.0 mL of water and stir evenly to obtain a clarithromycin drug-containing layer paste, which is loaded into the 3D printing barrel A for use.
[0066] 2: Take 4.2 g of water-insoluble polymer material-ethyl cellulose (EC 20), add it to 50 mL of absolute ethanol, stir until dissolved, then add inorganic carbonate (0.40 g of sodium bicarbonate powder), stir at high speed to disperse evenly. 1.40 g of sodium carboxymethyl cellulose, a water-e...
Embodiment 2
[0071] A preparation method of a hollow clarithromycin gastric floating tablet prepared based on a semi-solid 3D printing technology: the raw and auxiliary materials are respectively passed through a 90-mesh sieve.
[0072] 1: Take release modifier (PEG 4000 0.60g), binder (crospovidone 0.30g), water-erodible polymer skeleton material (carbomer 934p 0.70g), mix well, add 11mL of 95% ethanol, Stir well, and stir 6.80 g of clarithromycin micropowder until it solidifies. Crush the solidified product, slowly add 2.5 mL of water and stir evenly to obtain a clarithromycin drug-containing layer paste, which is loaded into the 3D printing barrel A for use.
[0073] 2: Take 2.0g of water-insoluble polymer skeleton material - Eudragit E PO, add it to 30mL of absolute ethanol, stir until dissolved, add inorganic carbonate (sodium bicarbonate powder 0.55g), stir at high speed to disperse evenly. Add 1.20 g of water-erodible polymer material-Carbomer 934p powder, and stir at high speed to...
Embodiment 3
[0078] A preparation method of a hollow clarithromycin gastric floating tablet prepared based on a semi-solid 3D printing technology: the raw and auxiliary materials are respectively passed through a 120-mesh sieve.
[0079]1: Take the release modifier (poloxamer P188 0.50g), the binder (hypromellose k30 0.40g), the water-erodible polymer skeleton material (chitosan 0.50g), mix well, add 95% ethanol 13mL, stir well, clarithromycin micropowder 7.2g, stir until solidified. Crush the solidified product, slowly add 3.5 mL of water and stir evenly to obtain a clarithromycin drug-containing layer paste, which is loaded into the 3D printing barrel A for use.
[0080] 2: Water-insoluble polymer skeleton material-ethyl cellulose (EC 100) 3.40g, add 60mL absolute ethanol, stir until dissolved, add inorganic carbonate (sodium bicarbonate powder 0.45g), stir at high speed to disperse evenly. 2.20 g of water-erodible polymer skeleton material-gelatin was added, and a suspension gel was f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com