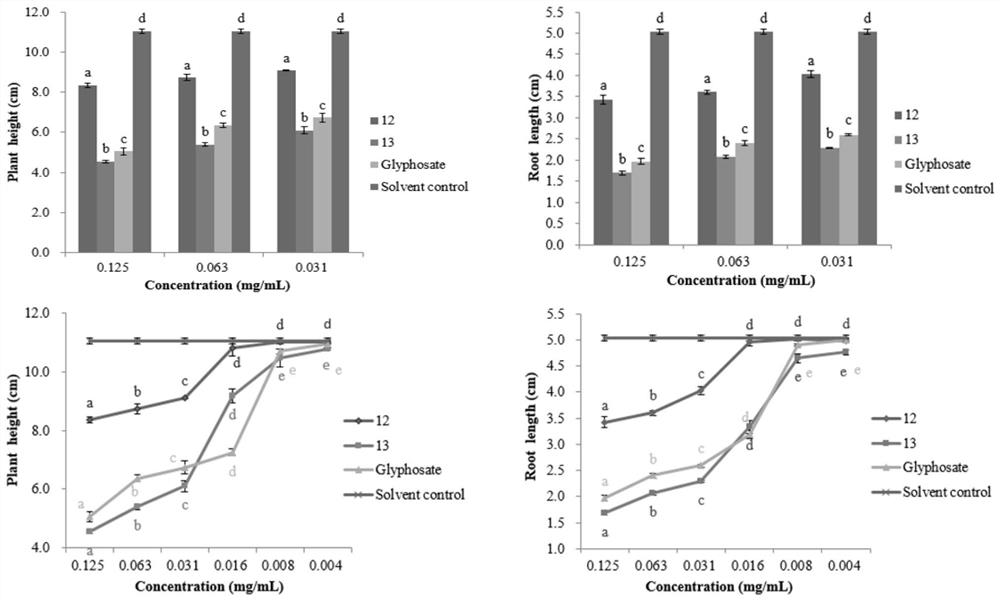
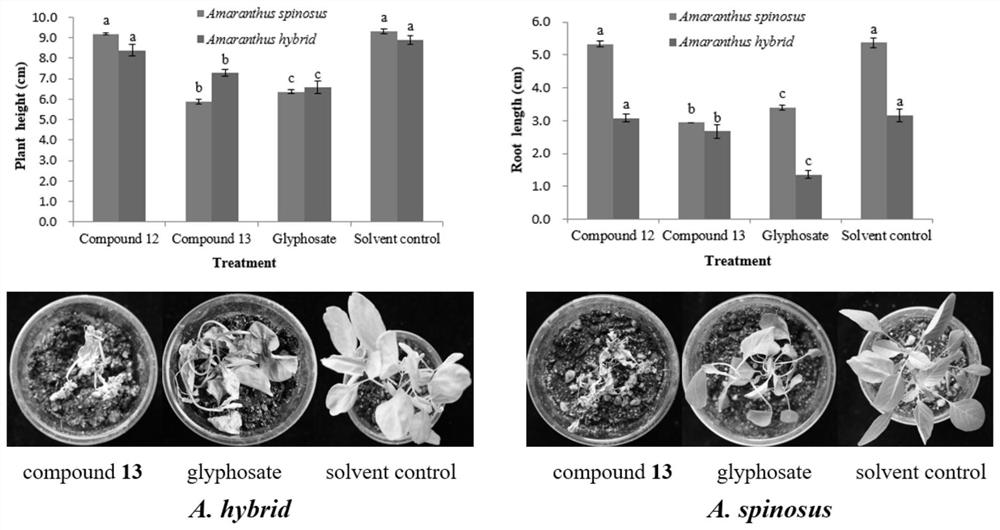
Xanthone compounds and preparation method thereof, and application of Xanthone compounds in field of agriculture
A technology of compounds and mixtures, applied in the field of fungal extracts, to achieve good inhibitory effect and excellent herbicidal performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 strain source
[0033] The embodiment of the present invention provides a herb-inhibiting fungus screened from seaweed. The herb-inhibiting fungus is Aspergillus versicolor D5. The date is February 6, 2018, and the preservation address is the Institute of Microbiology, Chinese Academy of Sciences, No. 3, Yard 1, Beichen West Road, Chaoyang District, Beijing.
[0034] The Aspergillus versicolor D5 was cultured on a PDA plate medium at 28° C. for 5 days, the diameter of the colony was 3 cm, the hyphae were orange-yellow, the surface was flocculent, and the edge was white. The conidia heads are radial at the beginning, the apical capsule is slightly elongated or slightly oval, the sporulation structure is double-layered, and the conidia are balls.
[0035] The 18S rDNA gene sequence of the Aspergillus versicolor D5 strain has been submitted to the GenBank database with the accession number MG827180 (since the gene sequence has been submitted to the GenBank datab...
Embodiment 2
[0036] Embodiment 2 fermentation culture obtains extract
[0037] Aspergillus versicolor D5 strain was grown on potato dextrose agar medium (PDA) for one week, edge hyphae were picked and added to the seed medium, and cultured at 28°C and 180rpm for 72h with shaking. Add 400mL potato dextrose water culture medium into a 1000mL fermentation bottle, after sterilization, absorb 5mL seed solution and add it to the culture medium, let it stand at 28°C for 30 days, and ferment 100L in total. It can be understood that the fermentation temperature of the strain can be around 28°C, and the specific fermentation time can be shortened or extended according to the fermentation situation.
[0038] Among them, potato dextrose agar (PDA) medium: potato extract powder 5-6g / L, glucose 15-20g / L, coarse sea salt 25-30g / L, and the rest is deionized water. It can be understood that the ratio of potato and glucose in the medium is optimized and selected for Aspergillus versicolor D5, which can eff...
Embodiment 3
[0041] Example 3 compound separation
[0042] The obtained fermented extract is processed sequentially by using ethyl acetate-petroleum ether solvent system (the elution ratio of ethyl acetate is 0-100%) and methanol-ethyl acetate solvent system (the elution ratio of methanol is 0-50%) The normal phase decompression column chromatography separated to obtain seven components of components 1-7.
[0043] Gradient elution of reversed-phase silica gel column chromatography was performed on component 2, and the solvent was 50%-100% methanol-water to obtain component 2-1-component 2-5. Component 2-2 was purified by Sephadex LH-20 gel column chromatography (dichloromethane / methanol, v / v, 1:1), and then purified by high performance liquid chromatography (HPLC), the solvent was 65% methanol-water , to obtain compound 11. Components 2-3 were subjected to SephadexLH-20CC (dichloromethane / methanol, v / v, 1:1) gel column chromatography, and then ODS reversed-phase silica gel column chromat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com