Florfenicol synthesis method
A florfenicol and compound technology, applied in the field of drug synthesis, can solve problems such as difficulty in recycling, increased energy consumption and safety risks, and serious poisoning in wastewater treatment systems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
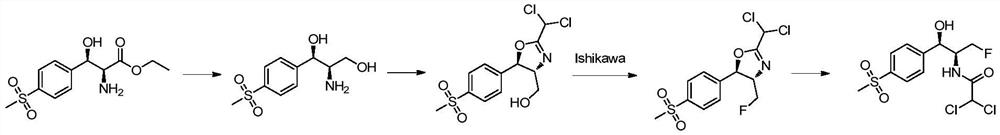
Examples
preparation example Construction
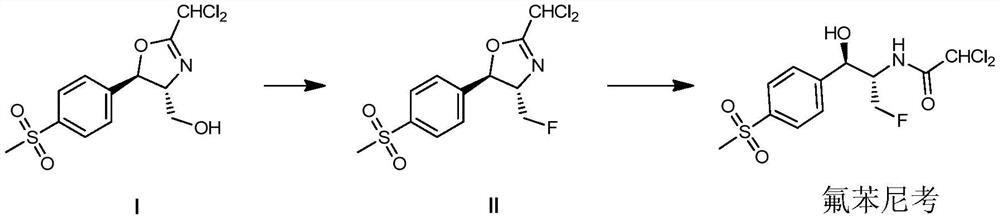
[0061] The invention provides a kind of preparation method of florfenicol, described method comprises steps:
[0062]
[0063] (a) In an inert solvent, in the presence of an organic base, carry out a fluorination reaction with compound I and sulfuryl fluoride, after the fluorination reaction is completed, the reaction mixture is concentrated, and the concentrate is collected without any separation, The purification step is used directly in the next step;
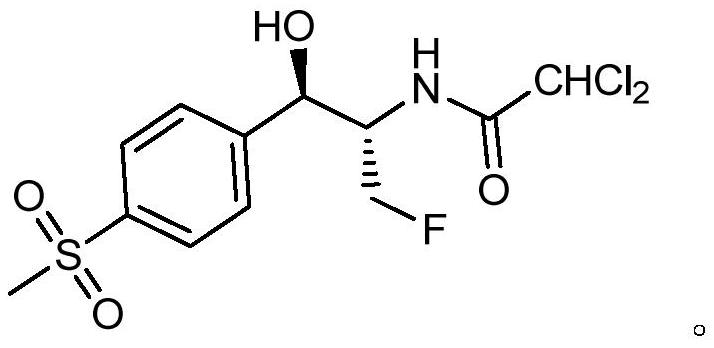
[0064] (b) In an aqueous system, subject the concentrate obtained in the preceding steps to a ring-opening reaction to obtain Florfenicol.
[0065] In another preferred example, in step (a), the ratio of compound I to inert solvent is 1 kg: 5-15 kg or liter; preferably 1 kg: 7-10 kg or liter.
[0066] In another preferred example, in step (a), the inert solvent is selected from the group consisting of acetonitrile, dichloromethane, dichloroethane, tetrahydrofuran or combinations thereof.
[0067] In another preferred ex...
Embodiment 1
[0107]
[0108] In a 500 mL reaction flask, compound I (1 equivalent), acetonitrile (8 volumes), and diisopropylethylamine (1.4 equivalents) were sequentially added. After the above mixture is stirred and lowered to -10 to -5 degrees Celsius, add sulfuryl fluoride balloons on the reaction bottle, and continue to react at a temperature of -10 to -5 degrees Celsius until the raw materials are no longer converted by HPLC (calculation of sulfuryl fluoride by balloon weight loss method). Fluorine consumption is about 1.3 equivalents). After the reaction mixture was concentrated to dryness under reduced pressure, 8 volumes of 25% isopropanol aqueous solution were added, the temperature was raised to 80°C and stirred for 3 hours, then cooled to 5-10°C, and filtered with suction to obtain the crude product of Florfenicol.
[0109] The crude product was heated and dissolved in 7 volumes of 30% isopropanol aqueous solution, then first cooled to 20-25°C, then cooled to 5-10°C and left...
Embodiment 2
[0111]
[0112]Get a 1000ml reaction kettle ready, equipped with a thermometer, add compound I (1 equivalent), dichloromethane (10 weights), diisopropylethylamine (1.2 equivalents) successively at room temperature, and control the temperature of the above mixture to 15 ~25 degrees Celsius, slowly introduce sulfuryl fluoride gas, the pressure is 2-3 atmospheres. Control the temperature at 15-25 degrees Celsius, react for 24 hours, and then concentrate the reaction mixture under reduced pressure to recover the solvent. Then add 8 volumes of 25% isopropanol aqueous solution into the reaction kettle, raise the temperature to 80-85 degrees Celsius, and stir for 3 hours. The reaction solution was cooled to 5-10 degrees Celsius and stirred for 1 hour. Suction filtration, the filter cake was washed with water and dried. Dry in a vacuum oven at 60-65°C for 16 hours to obtain a white solid, which meets the standards of the Pharmacopoeia of the People's Republic of China, with a yie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com