Antiviral compound containing luteolin and preparation method thereof
A kind of luteolin and antiviral technology, applied in the direction of antiviral agent, organic chemistry, etc., to achieve the effect of low cost and simple production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Preparation of antiviral luteolin-quercetin compound:
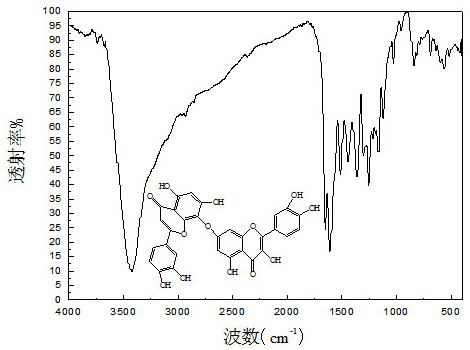
[0039] Step a: Dissolve NBS in acetone and stir to dissolve it at room temperature and avoid light; dissolve luteolin in acetone, add BPO, slowly add the acetone solution of NBS in an ice bath, and heat to 80°C, reacted for 18 hours, after the reaction was over, distilled under reduced pressure, evaporated acetone, washed with deionized water several times and freeze-dried to obtain 8-bromoluteolin, wherein n(luteolin):n(NBS )=1:1.5, m(BPO):m(luteolin)=5%;
[0040]Step b: Dissolve borax, 8-bromoluteolin and quercetin in water respectively in two three-necked flasks, and stir at room temperature for 1 hour to obtain 8-bromoluteolin boronic acid complex and quercetin boronic acid complex , wherein the molar ratio of n (8-bromoluteolin) to n (borax) is 7:1, and the molar ratio of n (quercetin) to n (borax) is 6:1;
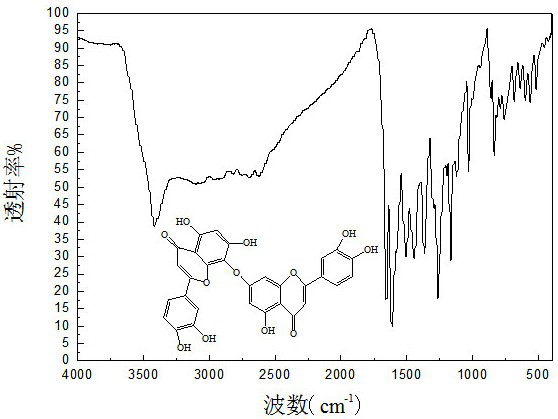
[0041] Step c: Add 8-bromoluteolin boronic acid complex, quercetin boronic acid complex, N-methylpyrr...
Embodiment 2
[0043] Preparation of antiviral luteolin-quercetin compound:
[0044] Step a: Dissolve NBS in acetone and stir to dissolve it at room temperature and avoid light; dissolve luteolin in acetone, add BPO, slowly add the acetone solution of NBS in an ice bath, and heat to 70°C, reacted for 12 hours, after the reaction was over, distilled under reduced pressure, evaporated acetone, washed with deionized water several times and freeze-dried to obtain 8-bromoluteolin, wherein n(luteolin):n(NBS )=1:1.1, m(BPO):m(luteolin)=4%;
[0045] Step b: Dissolve borax, 8-bromoluteolin and quercetin in water respectively in two three-necked flasks, and stir at room temperature for 1 hour to obtain 8-bromoluteolin boronic acid complex and quercetin boronic acid complex , wherein the molar ratio of n (8-bromoluteolin) to n (borax) is 5:1, and the molar ratio of n (quercetin) to n (borax) is 4:1;
[0046] Step c: Add 8-bromoluteolin boronic acid complex, quercetin boronic acid complex, N-methylpyr...
Embodiment 3
[0050] Preparation of antiviral luteolin-quercetin compound:
[0051] Step a: Dissolve NBS in acetone and stir to dissolve it at room temperature and avoid light; dissolve luteolin in acetone, add BPO, slowly add the acetone solution of NBS in an ice bath, and heat to 50°C, reacted for 9 hours, after the reaction was over, distilled under reduced pressure, evaporated acetone, washed several times with deionized water and freeze-dried to obtain 8-bromoluteolin, wherein n(luteolin):n(NBS )=1:1.1, m(BPO):m(luteolin)=2%;
[0052] Step b: Dissolve borax, 8-bromoluteolin and quercetin in water respectively in two three-necked flasks, and stir at room temperature for 1 hour to obtain 8-bromoluteolin boronic acid complex and quercetin boronic acid complex , wherein the molar ratio of n (8-bromoluteolin) to n (borax) is 3:1, and the molar ratio of n (quercetin) to n (borax) is 2:1;
[0053] Step c: Add 8-bromoluteolin boronic acid complex, quercetin boronic acid complex, N-methylpyrr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com