Lacosamide oral solution and preparation method thereof
A technology for lacosamide and oral solution, which is applied in the field of pharmaceutical preparations, can solve the problems of difficulty in preparing oral solution, complex preparation process, many preparation impurities, etc., and achieves the effects of simple and efficient preparation process, stable properties and improved solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
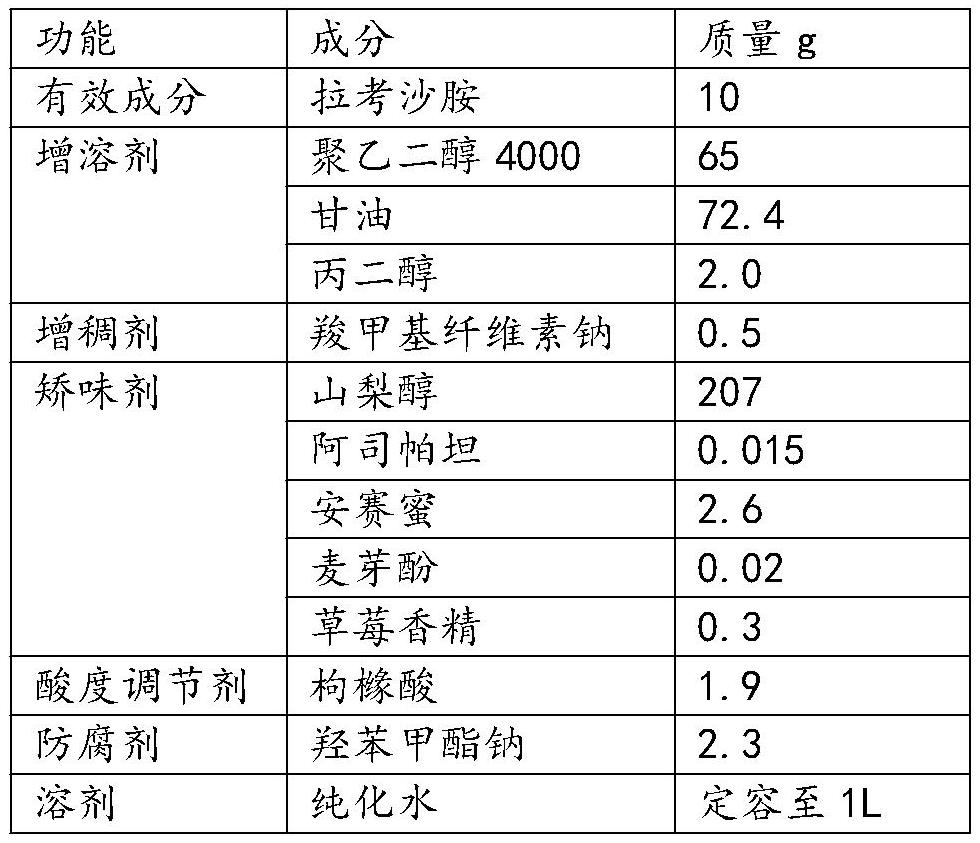
[0030] This embodiment provides a lacosamide oral solution, which is prepared by including the raw materials in Table 1.
[0031] Lacosamide oral solution components in Table 1 Example 1
[0032]
[0033] The preparation method of the lacosamide oral solution of the present embodiment comprises the following steps:
[0034] S1, add 450g purified water in the stirring tank, open stirring;
[0035] S2, after wetting the flavoring agent maltol with 3g purified water, put it into the tank, and stir until it is completely dissolved;
[0036] S3, the thickening agent sodium carboxymethyl cellulose added slowly is stirred until dispersion and swelling are complete;
[0037] S4, the citric acid, sorbitol, aspartame, acesulfame potassium, macrogol 4000, glycerol, propylene glycol and sodium paraben added successively, stirring until dissolving completely;
[0038] S5, add lacosamide, add purified water to 950 mL, and stir until dissolved completely;
[0039] S6, add strawberry e...
Embodiment 2
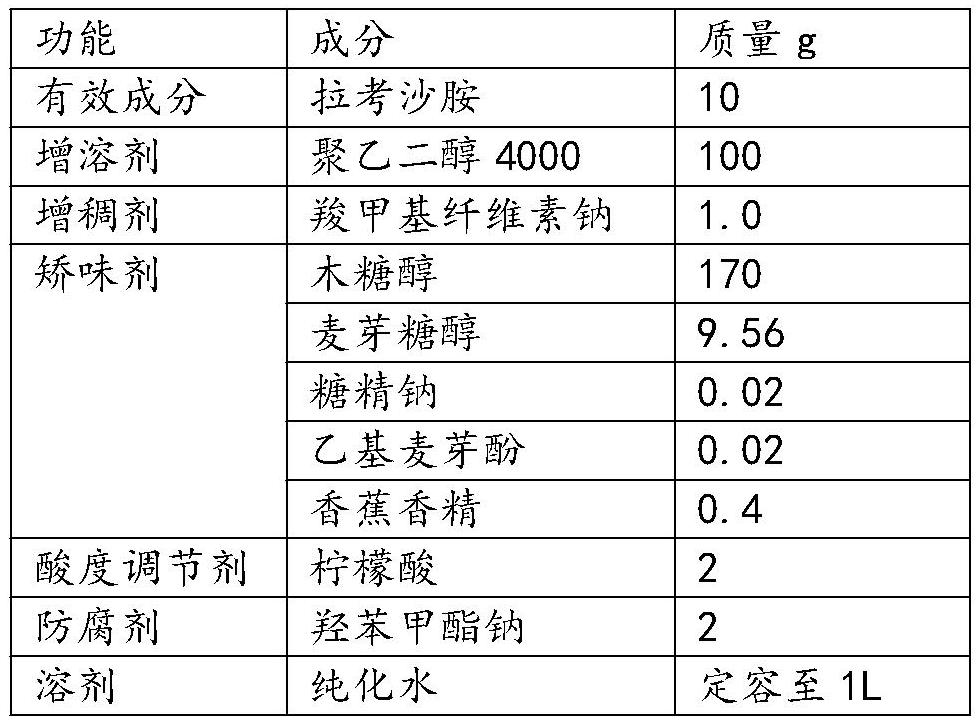
[0044] This embodiment provides a lacosamide oral solution, which is prepared by including the raw materials in Table 2.
[0045] Table 2 Lacosamide oral solution components in Example 2
[0046]
[0047] The preparation method of the lacosamide oral solution of the present embodiment comprises the following steps:
[0048] S1, add 450g purified water in the stirring tank, open stirring;
[0049] S2, after wetting the ethyl maltol with 3g of purified water, put it into the tank, and stir to dissolve completely;
[0050] S3, the thickening agent sodium carboxymethyl cellulose added slowly is stirred until dispersion and swelling are complete;
[0051] S4, the citric acid, maltitol, xylitol, sodium saccharin, polyethylene glycol 4000, sodium paraben added successively are stirred to dissolve completely;
[0052] S5, add lacosamide, add purified water to 950 mL, and stir until dissolved completely;
[0053] S6, add banana essence, and set the volume to 1L;
[0054] S7, fi...
Embodiment 3
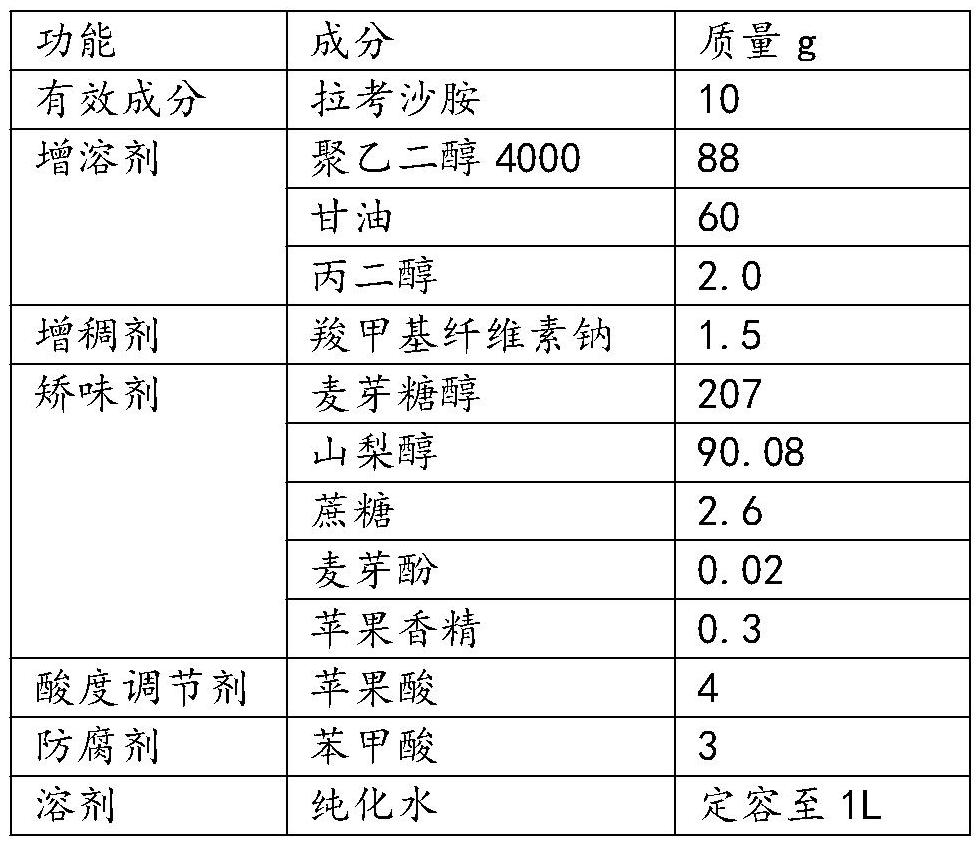
[0058] This embodiment provides a lacosamide oral solution, which is prepared from the raw materials included in 3.
[0059] Table 3 Lacosamide oral solution components in Example 3
[0060]
[0061] The preparation method of the lacosamide oral solution of the present embodiment comprises the following steps:
[0062] S1, add 450g purified water in the stirring tank, open stirring;
[0063] S2, after wetting the maltol with 3g of purified water, put it into the tank, and stir until it is completely dissolved;
[0064] S3, slowly add sodium carboxymethyl cellulose, and stir until dispersion and swelling are complete;
[0065] S4, the malic acid, maltitol, sorbitol, sucrose, macrogol 4000, glycerol and propylene glycol, benzoic acid added successively are stirred to dissolve completely;
[0066] S5, add lacosamide, add purified water to 950 mL, and stir until dissolved completely;
[0067] S6, add apple essence, make up to 1L;
[0068] S7, filter;
[0069] S8, filling;...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com