A tetraphenylethylene terpyridine organic ligand compound, coordination supramolecule and its preparation and application
A technology of terpyridine and tetraphenylethylene, applied in organic chemistry, chemical instruments and methods, luminescent materials, etc., can solve the problems of difficult separation and purification, many reaction steps, and difficult synthesis, and achieve low synthesis difficulty and mild reaction conditions , the effect of improving yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
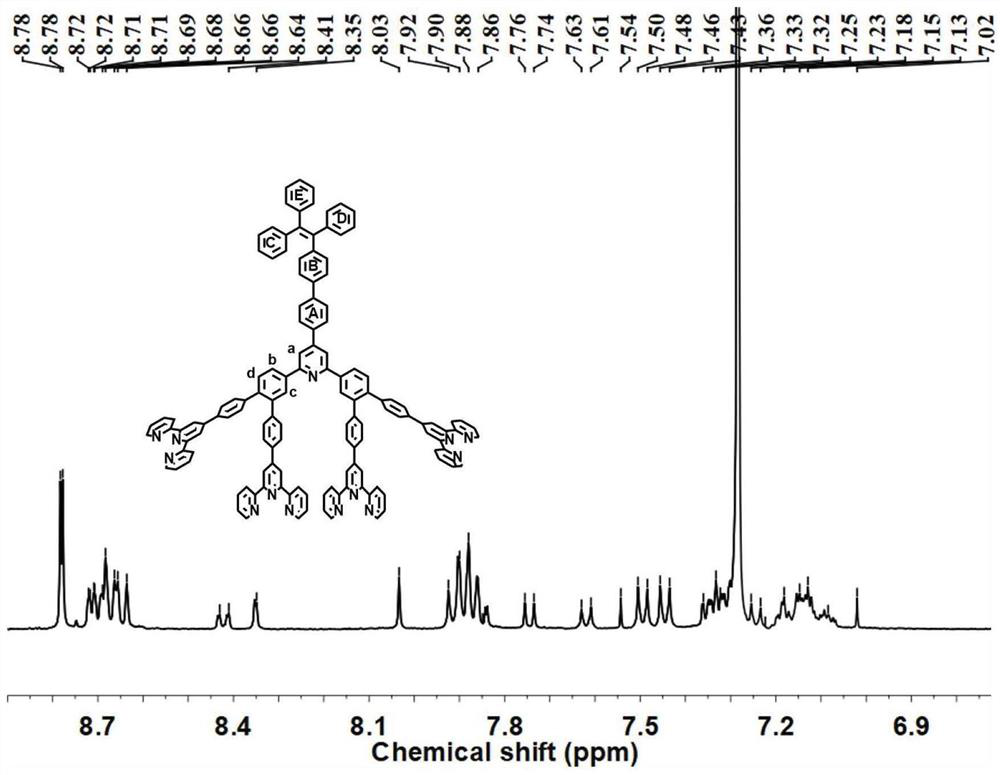
[0081] Step (1) [1-(4-formaldehyde phenyl)-1,2,2-triphenyl]ethylene (500mg, 1.39mmol) and 3,4-dibromoacetophenone (930mg, 3.32mmol) Add it into a 250mL round bottom flask containing 100mL / 10mL absolute ethanol / toluene, then add NaOH (166mg, 4.17mmol), and stir overnight at room temperature. Add 20mL of ammonia water to the mixed reaction solution, add reflux for 10h, wait for the reaction solution to cool to room temperature, filter to obtain a solid, rinse with isopropanol to obtain a white solid L (1060mg), the yield is 80%. 1 H NMR (500MHz, CDCl 3 )δ8.41(s,2H,H a ),7.98-7.97(d,2H,H b ),7.83(s,2H,H c ),7.78-7.76(d,2H,H d ),7.52-7.50(d,2H,tpy-H g ),7.23-7.21(d,2H,tpy-H h ),7.17-7.07(m,19H, B,C,D,E- Ph-H).
[0082] Step (2) Add compound L (1000mg, 1.05mmol), 4-phenylboronic acid-2,2':6,2"-terpyridine (1780mg, 5.04mmol), NaOH (504mg, 12.60 mmol) and solvent THF / DMF (120:20mL), then add Pd(Ph 3 ) 4 (290mg, 0.25mmol, under the protection of nitrogen, reflux overnight a...
Embodiment 2
[0090] to containing 60mLCH 2 Cl 2 In a 100mL round bottom flask, add o-dimethoxybenzene (2g, 14.47mmol), after the o-dimethoxybenzene is completely dissolved, add liquid bromine (5.1g , 31.84mmol) of dichloromethane solution 10mL, the mixed solution was reacted at room temperature for 2h. After the reaction was completed, add a saturated aqueous solution of sodium bisulfite to quench the unreacted liquid bromine, then extract three times with water and saturated experimental water, collect the lower organic solvent, and spin dry to obtain a white solid 3 (4.2g,), The yield was 98%. 1 HNMR (500MHz, CDCl 3 )δ7.08(s,2H,ph-H),3.88(s,6H,-OCH 3 ).
[0091] Add compound 3 (3g, 10.14mmol), 4-phenylboronic acid-2,2':6,2"-terpyridine (8.5g, 24.33mmol) in a 250mL round bottom flask, NaOH (2.4g, 60.82mmol) and solvent THF (80mL), then add Pd(Ph 3 ) 4 (1.4g, 1.22mmol), under the protection of nitrogen, reflux at 80°C overnight. After the reaction was completed, the reaction liqui...
Embodiment 3
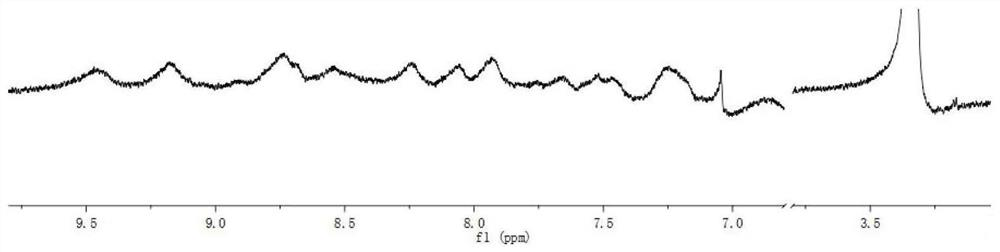
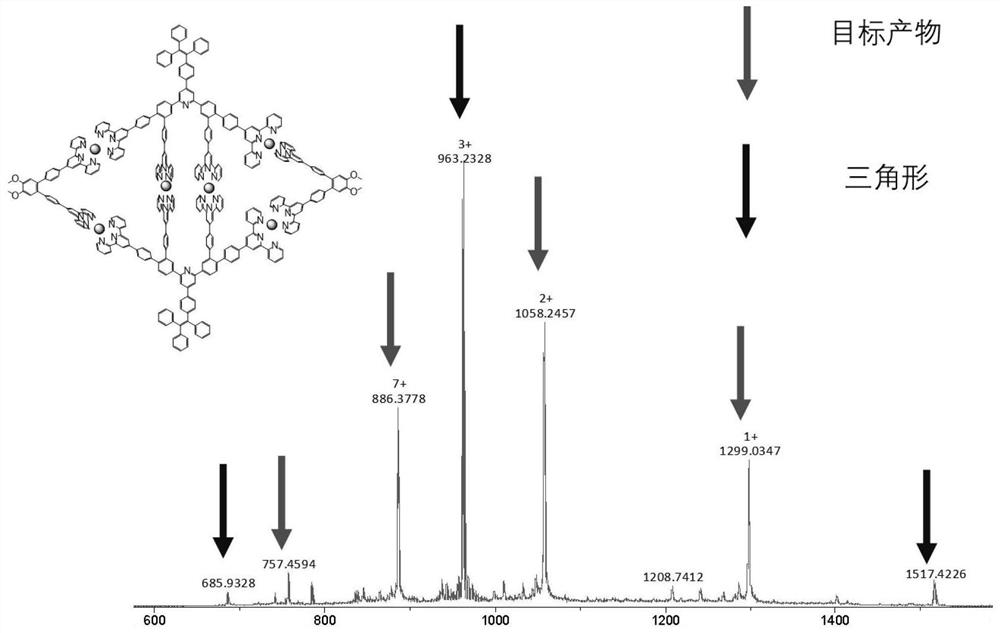
[0093] Add ligand V (10mg, 0.013mmol) and ligand R (23.8mg, 0.013mmol) to a 100mL round bottom flask, then add CH 3 OH / CH 3 CN / CHCl 3 (20mL / 20mL / 20mL), sonicate for 10min, and after the two ligands are completely dissolved, add Zn(NO 3 ) 2 Methanol solution (2mL, 49mg / 8mL), react overnight at 55°C. After the reaction was completed, the reaction liquid was cooled to room temperature, ammonium hexafluorophosphate was added and stirred for 30 minutes, and the yellow solid C 1 , yield 92%. 1 HNMR spectrum see Figure 5 , ESI-MS spectrum see Figure 6 . See NMR for purity. The complex mainly proves whether it is formed. NMR can characterize the formation and purity to a certain extent. ESI-MS can prove the existence and structural characteristics of the product.
[0094] 1 H NMR (500MHz, CD 3 CN):δ9.10-9.05(m,12H, 1,2,3-tpy- h 3',5' ),8.83-8.81(m,20H, 2,3-tpy- h 4',4” ,H b,c ),8.64-8.63(m,20H, 1-tpy- h 4',4” ),8.24-8.11(m,44H, 1,2-tpy- h g , 1,3-tpy- h 5 ',5” ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com