Application of magnesium isoglycyrrhizinate to preparation of medicine for treating colitis
A kind of technology of magnesium isoglycyrrhizinate and colitis, applied in the application field of magnesium isoglycyrrhizinate in the preparation of medicine for treating colitis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
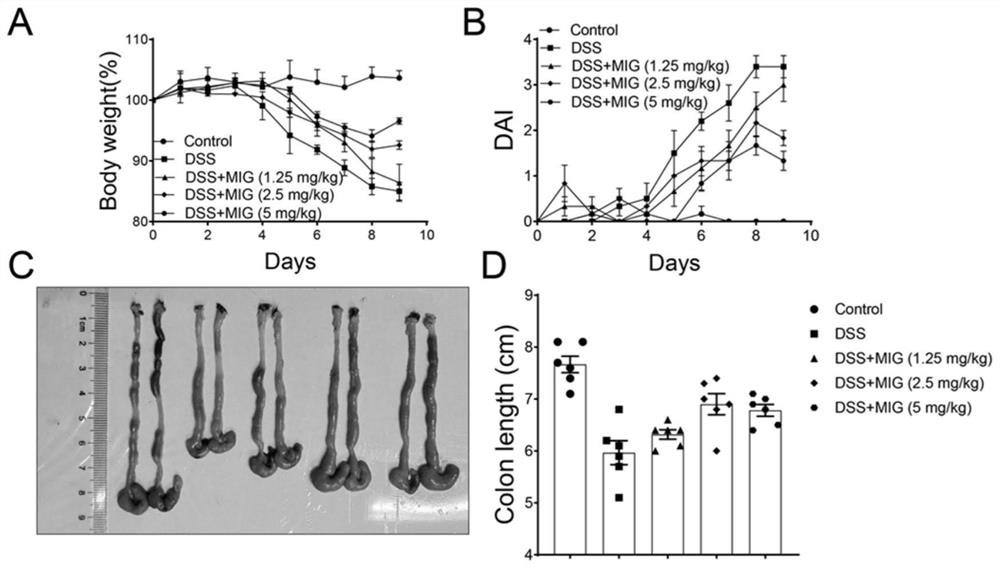
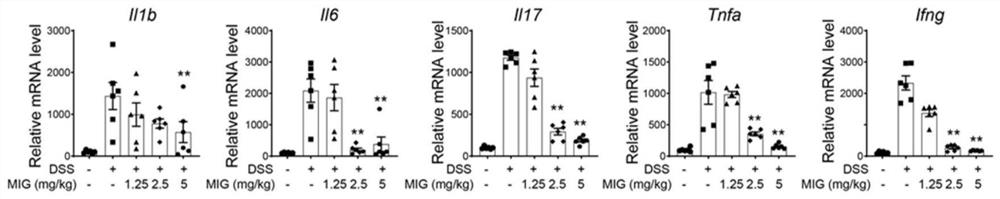
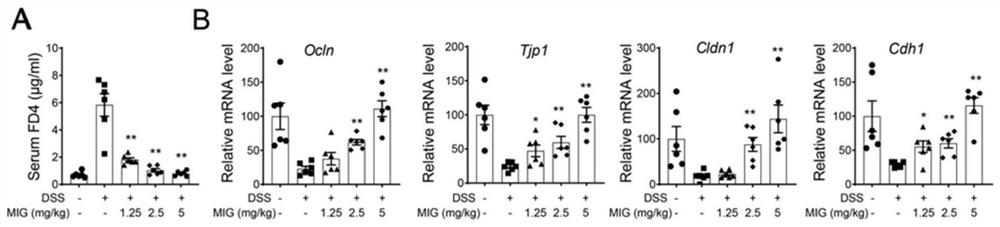
[0023] 1. Pharmacological experiment of magnesium isoglycyrrhizinate injection: DSS-induced colitis model in mice[1]
[0024] C57BL / 6 female mice, aged 6-8 weeks, weighing 22-24 g, were raised in an SPF animal room at 21±2°C, with free access to water and food, and alternated day and night for 12 hours. Mice were randomly divided into 5 groups, normal group (Sham group), model group (DSS group), magnesium isoglycyrrhizinate (Magnesium Isoglycyrrhizinate, MIG) low, medium and high dose groups (1.25mg / kg, 2.5mg / kg, 5mg / kg kg). The mice in the model group and the administration group were fed with 2.5% DSS for 7 days, followed by 2 days of water recovery. The administration group was intraperitoneally administered according to the dose, once a day, 200 μl each time. Afterwards, the body weight of the mice was recorded every day and the disease activity index (DAI) was scored, and the mice were sacrificed on the 9th day, and then the length of the colon and histopathological sec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com