Spectinomycin and lincomycin soluble powder capable of being completely dissolved in various solvents and preparation method
A spectinomycin, complete dissolution technology, applied in non-active ingredient medical preparations, medical preparations containing active ingredients, powder delivery, etc., can solve severe pain, animal stress response, increase injection dose and other problems, to achieve the effects of strong antibacterial spectrum and activity, repair of cell membrane damage, and stable dispersion properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 15
[0035] Example 15: Appearance stability of spectinomycin lincomycin soluble powder powder involved in the present invention
[0036] Test spectinomycin lincomycin soluble powder: prepared by the methods of Comparative Examples 1-5 and Examples 1-14.
[0037] Test conditions:
[0038] (1) High-temperature, high-humidity test: The sample is placed in a closed container with constant humidity, and placed at 60°C for 10 days at a relative humidity of 95%±5%, and samples are taken on the 5th and 10th days.
[0039] (2) Accelerated test: place for six months at a temperature of 40°C±2°C and a humidity of 75%±5%, and take samples at the end of the first month, second month, third month, and sixth month.
[0040] The test results are shown in Table 2:
[0041] Note: " / " means no test
[0042] Table 2
[0043]
[0044]
Embodiment 16
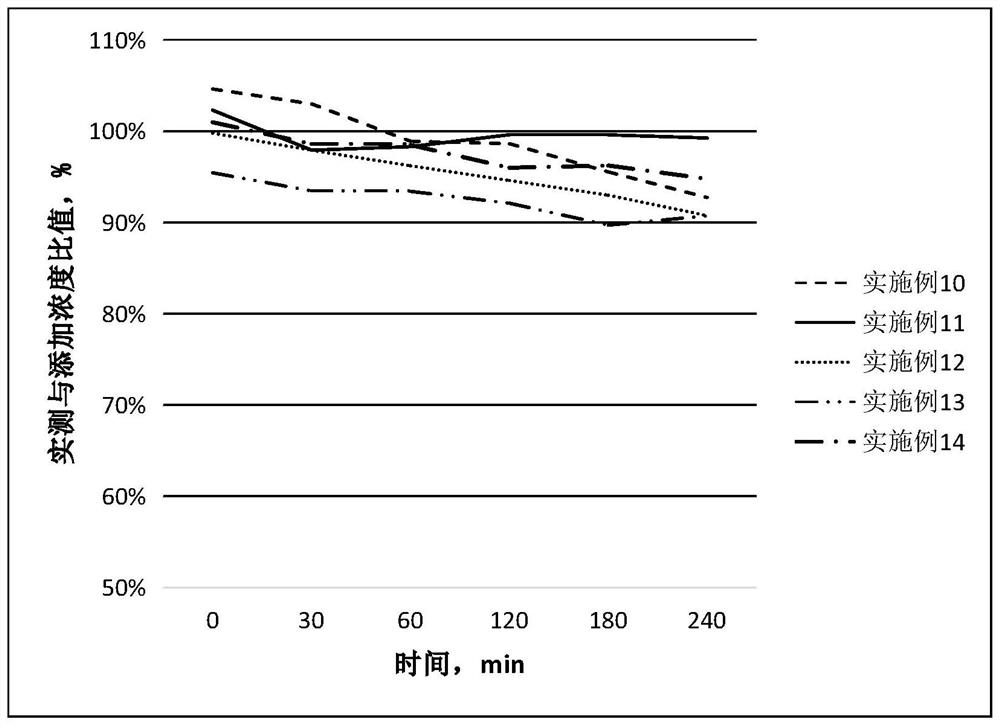
[0045] Example 16: Stability Test of Spectinomycin Lincomycin Soluble Powder Dissolved in Various Solvents
[0046] Test spectinomycin lincomycin soluble powder: prepared by the methods of Comparative Example 1-5, Example 2, and 5-14.
[0047] Seedlings: Recombinant avian influenza virus (H5+H7) trivalent inactivated seedlings (H5N1 Re-11 strain + Re-12 strain, H7N9 H7-Re2 strain): batch number 01924058, Zhaoqing Dahuanong Biopharmaceutical Co., Ltd.
[0048] Marek's Diluent: Chicken Marek's Disease Vaccine Diluent: Lot No. S18041903, Merial Animal Health Ltd.
[0049] Test conditions: (1) Add 10g of spectinomycin lincomycin soluble powder to 30ml of water, 0.9% sodium chloride solution, and Marek diluent at 25°C, and observe the dissolution within 5 minutes;
[0050] (2) At 25°C, add 40g of spectinomycin lincomycin soluble powder to 100ml of water, 0.9% sodium chloride solution, and Marek diluent, and observe the dissolution within 5 minutes;
[0051] (3) Add 10 g of specti...
Embodiment 17
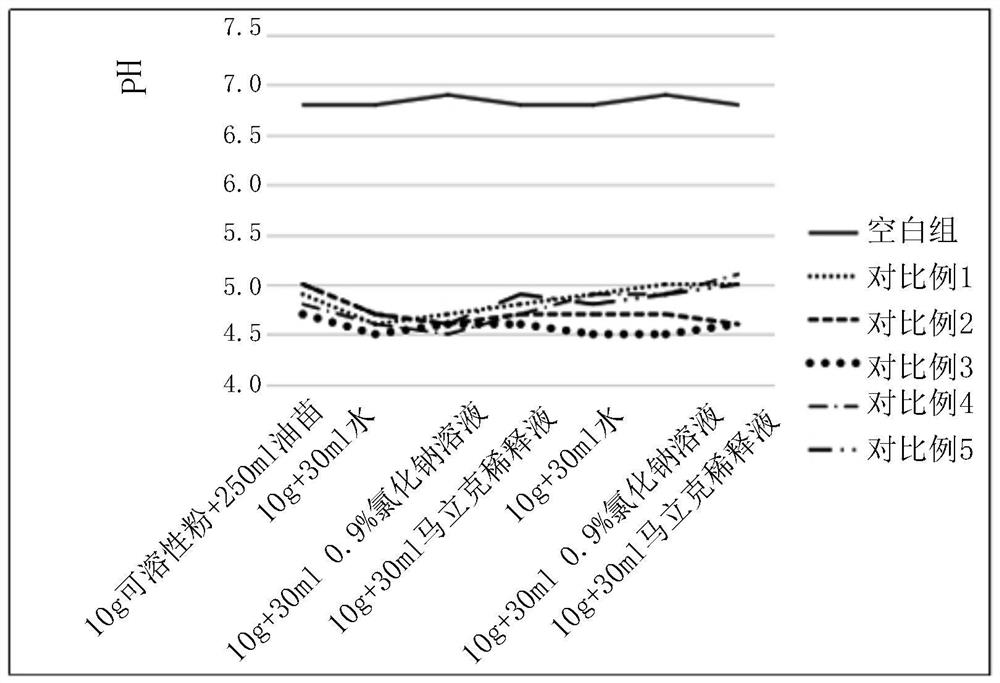
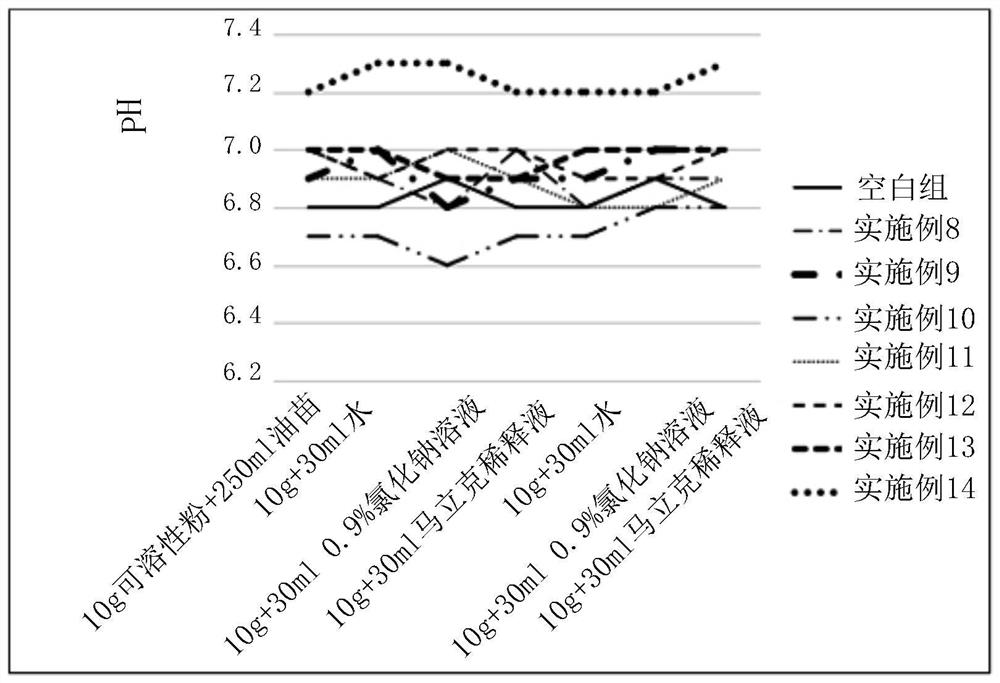
[0056] Embodiment 17: The pH test situation of spectinomycin lincomycin soluble powder dissolved in various solvents involved in the present invention
[0057] Test spectinomycin lincomycin soluble powder: prepared by the methods of Comparative Examples 1-5 and Examples 8-14.
[0058] Seedlings: Recombinant avian influenza virus (H5+H7) trivalent inactivated seedlings (H5N1 Re-11 strain + Re-12 strain, H7N9 H7-Re2 strain): batch number 01924058, Zhaoqing Dahuanong Biopharmaceutical Co., Ltd.
[0059] Marek's Diluent: Chicken Marek's Disease Vaccine Diluent: Lot No. S18041903, Merial Animal Health Ltd.
[0060] Test conditions: (1) 10g spectinomycin lincomycin soluble powder was added to 30ml water, 0.9% sodium chloride solution, Marek diluent, and the pH of the solution was tested at a temperature of 25°C;
[0061] (2) Add 40 g of spectinomycin lincomycin soluble powder to 100 ml of water, 0.9% sodium chloride solution, and Marek diluent, and test the pH of the solution at a te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com