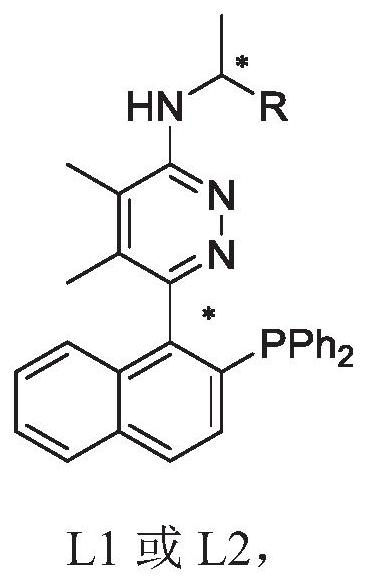
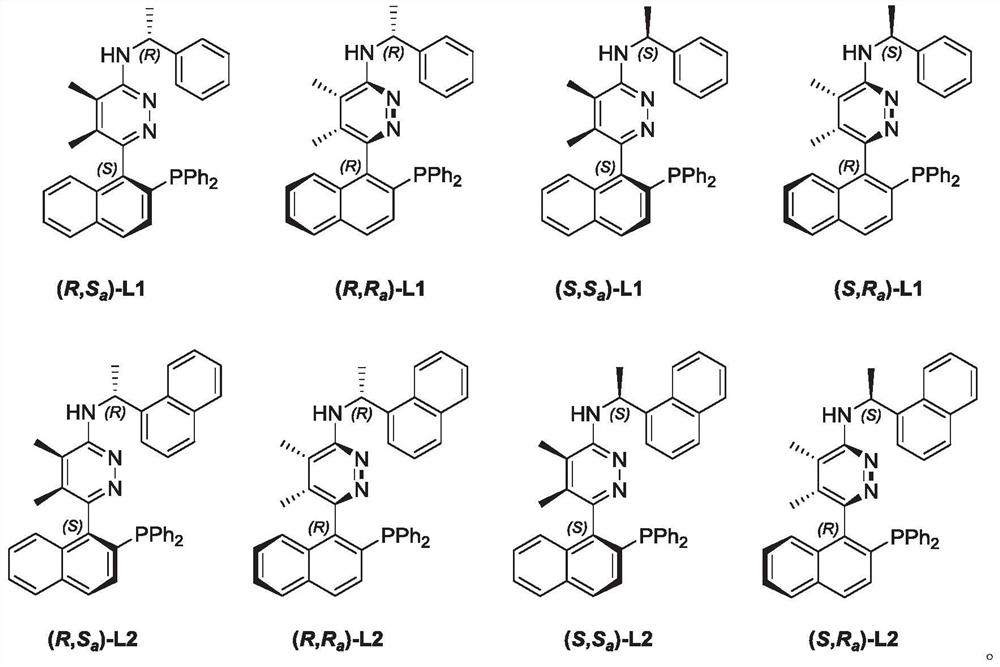
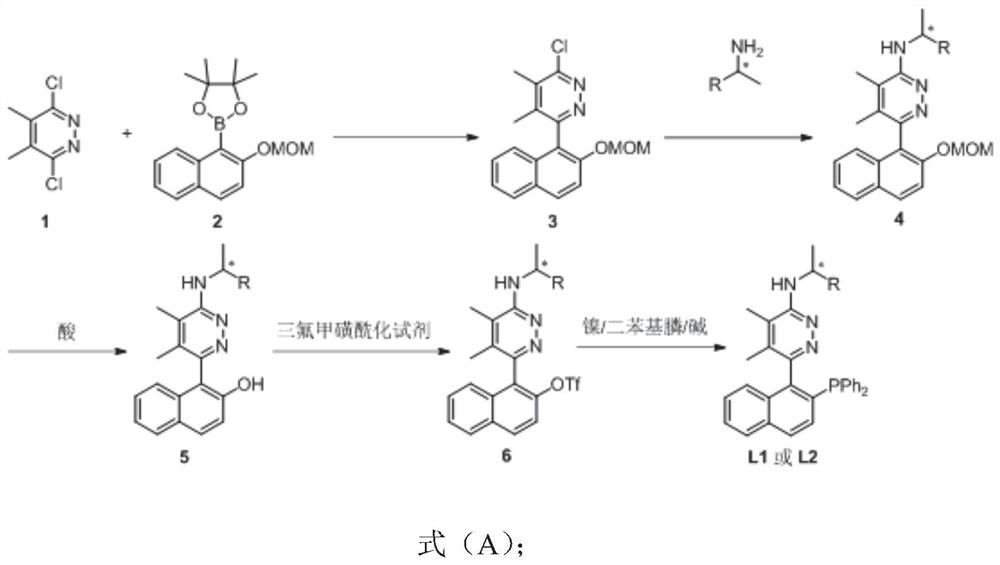
Phosphine nitrogen ligands with various chiral centers as well as synthesis method and application thereof
A technology of chiral centers and phosphine ligands, applied in organic chemical methods, organic compound/hydride/coordination complex catalysts, chemical instruments and methods, etc., can solve the problems of low reactivity and long reaction time of aromatic aldehydes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076]
[0077] Add 1 (2.7111g, 15mmol), 2 (6.6024g, 21mmol), Pd(OAc) successively in the reaction flask with reflux condenser 2 (170.5mg, 0.75mmol), PPh3 (787.5mg, 3mmol), and Na 2 CO 3 (3.2155 g, 30 mmol). The reaction flask was replaced with Ar three times, and 44 mL of a mixed solvent (ethylene glycol dimethyl ether: water = 3:1) was added. The device was placed in an oil bath preheated to 120°C, stirred and refluxed for 24 hours, and the reaction was detected by TLC. Remove from the oil bath, cool to room temperature naturally, add 18mL of water, and extract with 3×40mL dichloromethane. The organic phases were combined, dried over anhydrous sodium sulfate, filtered and concentrated. Column chromatography (eluent: petroleum ether / ethyl acetate = 10:1 (550 mL) to 5:1 (1560 mL) to 4:1 (250 mL)) gave the product 3 (2.8810 g, 58%) as a white solid: mp 131.9-132.6°C (ethyl acetate / petroleum ether); 1 H NMR (400MHz, CDCl 3 )δ7.96(d, J=9.2Hz, 1H, ArH), 7.86(d, J=9.2Hz, ...
Embodiment 2
[0079]
[0080] Under the protection of inert gas, add Pd(OAc) successively to the dry reaction bottle with reflux condenser 2(46.0 mg, 0.2 mmol), rac 1,1'-binaphthyl-2,2'-bisdiphenylphosphine (191.3 mg, 0.3 mmol) and toluene (4 mL). After stirring at room temperature for 30 minutes, 3 (1.3188g, 4mmol), (R)-1-phenyl-1-ethylamine (0.68mL, d=0.940g / mL, 0.6392g, 5.2mmol) / toluene (4mL) were added successively ) and Cs 2 CO 3 (1.8293 g, 5.6 mmol). The mixture was stirred and refluxed in an oil bath preheated to 120° C. for 9 hours, after the completion of the reaction as detected by TLC. The oil bath was removed, cooled to room temperature naturally, the mixture was filtered through a short basic aluminum oxide column (200-300 mesh), and rinsed with 80 mL of ethyl acetate. The filtrate was concentrated, and column chromatography (eluent: petroleum ether / ethyl acetate=4:1 (750mL) to 3:1 (1200mL) to 2:1 (750mL)) gave a yellow solid foam (R, S a )-4a and (R, R a )-4a mixture ...
Embodiment 3
[0082]
[0083] Add (R, S a )-4a and (R,R a )-4a mixture (d.r.=1:1, 887.5mg, 2.15mmol) and 42.9mL HCl (3M MeOH / H 2 O mixed solution). The mixture was stirred at room temperature for 15.5 h, after completion of the reaction as detected by TLC. Add concentrated ammonia water to neutralize to neutral, add 20 mL of water to dilute, and extract with 3×40 mL of dichloromethane, combine the organic phases, dry over anhydrous sodium sulfate, filter, concentrate to obtain a yellow solid foam, which is directly used in the next step without purification.
[0084] The crude product obtained in the previous step and 4-dimethylaminopyridine (26.7 mg, 0.215 mmol) were added to the reaction flask. The reaction flask was replaced with Ar three times, and 21.5 mL of DCM, Et 3 N (0.30 mL, d=0.728 g / mL, 0.2184 g, 2.15 mmol), and N-phenylbis(trifluoromethanesulfonyl)imide (784.3 mg, 2.15 mmol). The mixture was stirred at room temperature for 23.5 hours after completion of the reaction by ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com