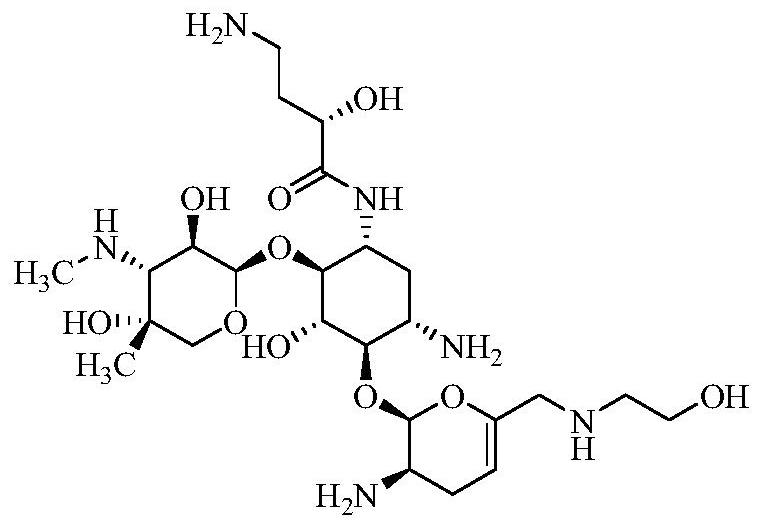
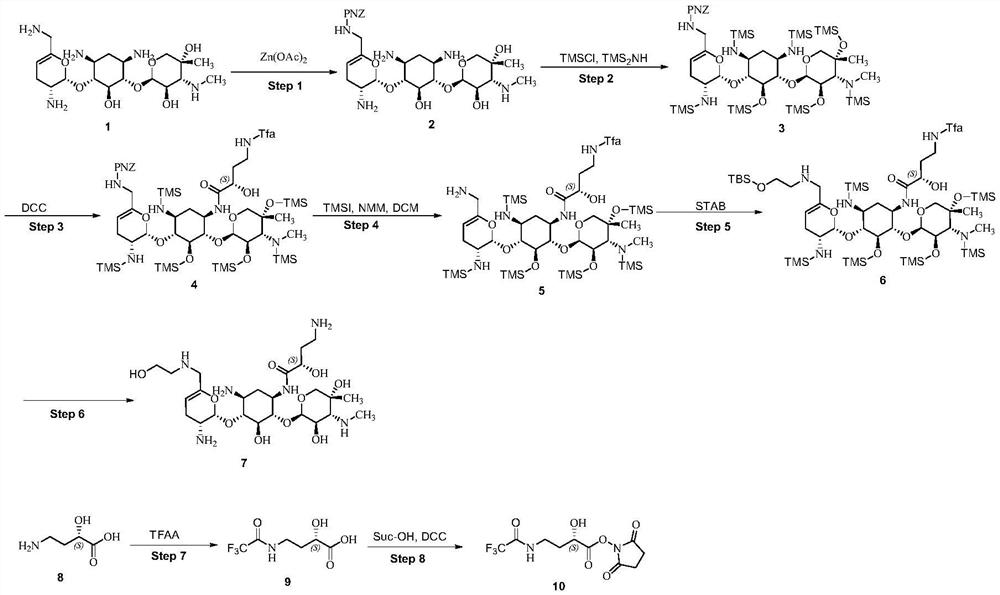
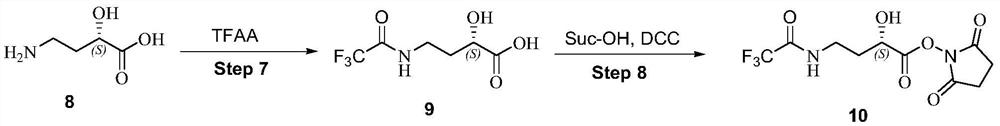
A kind of preparation method of plazomicin
A compound and selective technology, applied in the preparation of sugar derivatives, chemical instruments and methods, organic chemistry, etc., can solve the problems of incomplete literature reports, few synthesis steps, poor selectivity, etc., achieve simple post-processing steps, improve yield rate, the effect of increasing selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: the preparation of active ester
[0036]
[0037] 1) Preparation of compound 9
[0038] Potassium carbonate (29.0 g, 210 mmol) was dissolved in water (240 mL), compound 8 (10.0 g, 84.0 mmol) was added, and stirred for 30 min. Then a solution of trifluoroacetic anhydride (21.2 g, 100.8 mmol) in dioxane (112 mL) was added dropwise, and reacted overnight at room temperature. Part of the solvent was evaporated, water (300 mL) was added, and washed with methyl tert-butyl ether (50.0 mL×3). The aqueous layer was washed with KHSO under ice bath4 The pH of the solution (2M) was adjusted to 3-4, adding saturated brine (350mL), extracting with DCM (300mL×4), drying the organic phase with anhydrous sodium sulfate, filtering, and concentrating the filtrate to dryness to obtain an oily substance, that is, compound 9 (14.0 g, 77.2%).
[0039] 2) Preparation of Compound 10
[0040] The above oil (12.0g, 55.8mmol) was dissolved in 100ml of dichloromethane, and N-hy...
Embodiment 2
[0041] Embodiment 2: Preparation of Plazomicin
[0042] 1) Preparation of Compound 2
[0043] Dissolve p-nitrobenzyloxycarbonyl chloride (26.5g, 113mmol) in tetrahydrofuran (THF, 450mL), cool down to 0°C, add N-hydroxy-5-norbornene-2,3-dicarboximide (20.0g, 111.6mmol), then a solution of triethylamine (23.0mL, 167mmol) in THF (250mL) was added dropwise, after the dropwise addition, it was moved to room temperature for 6h. After the reaction, the reaction liquid was lowered to -5~0°C, stirred for 1 h, filtered, and the filtrate was concentrated. The resulting solid was stirred in isopropyl ether (200 mL) for 2 h, and filtered to obtain a white solid (N-hydroxy-5-norbornene-2,3-dicarboximide)-4-nitro-benzyloxymethanol Ester (HONB-PNZ, 37.5 g, 95.1%).
[0044] Dissolve compound 1 (sisomicin, 35.8 g, 80.7 mmol) in methanol (255 mL), add zinc acetate (44.0 g, 239.7 mmol), and stir at 0°C for 24 h. The temperature was lowered to -20°C to -10°C, and the DCM (544mL) solution of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com