A preparation method of a bismuth-doped bimetallic sulfide electrode for electrocatalytic oxidation of urea
An electrocatalytic oxidation and bimetallic technology, applied in the field of nanomaterials, can solve the problem of high cost and achieve the effects of low raw material cost, good electrocatalytic performance and stability, high electrocatalytic activity and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
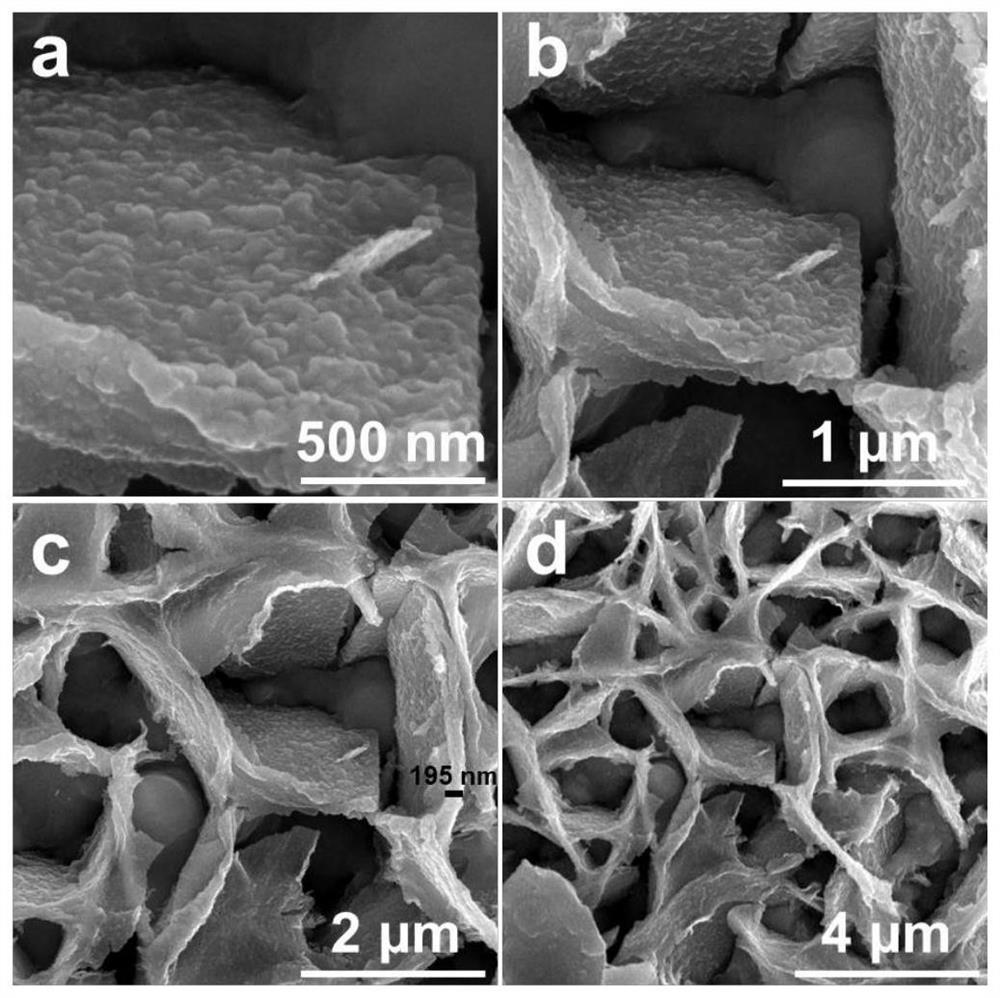
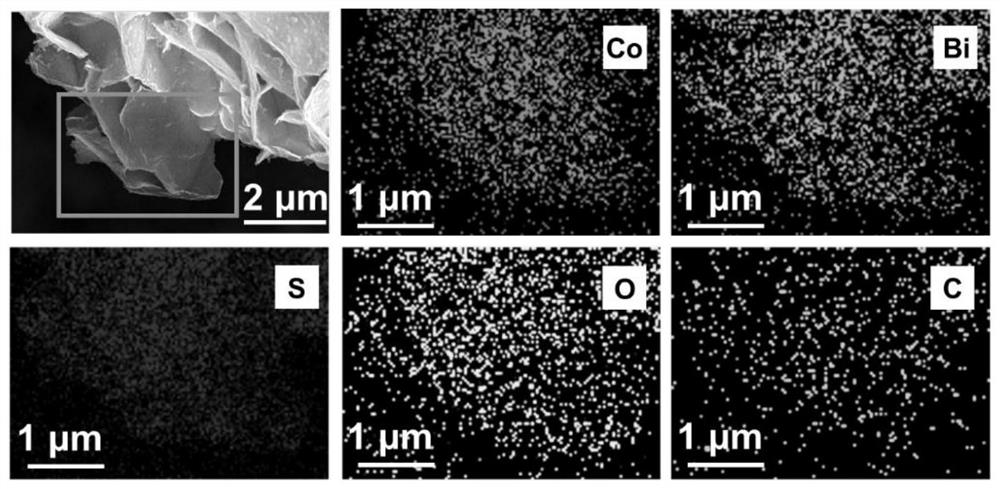
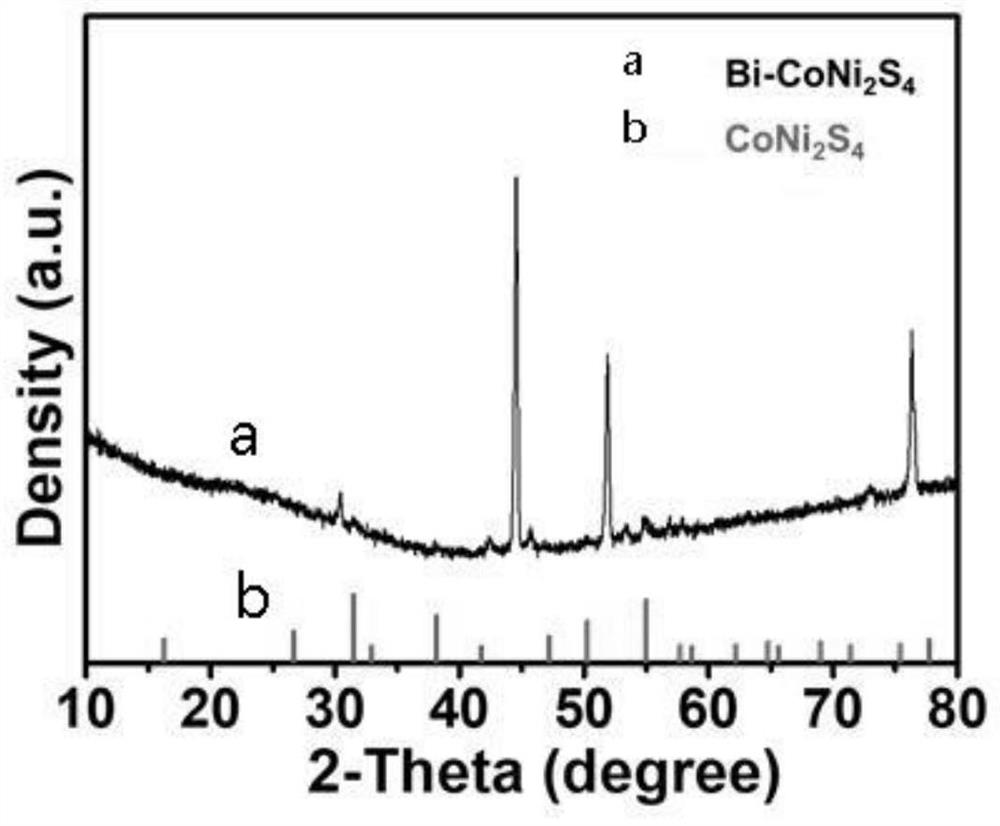
Image
Examples
Embodiment 1
[0030] (1) Cut 1 cm * 3 cm foam nickel, with dilute hydrochloric acid, ethanol, deionized water to clean each 5 minutes, spare;
[0031] (2) Take a clean beaker, add 25ml to ion water, weigh 0.043 g Na 2 Bio 4 · 2h 2 O, 0.36g CO (NO 3 ) 2 · 6h 2 O and 0.36G Ni (NO 3 ) 2 · 6h 2 O Pour in deionized water, weigh 0.24 g 2-methylimidazole into a metal salt mixture, ultrasonic dissolution, pour in a 50 mL reaction kettle; the foam nickel after pre-treatment after step (1) is added to the reaction In the kettle, place the oven to 120 ° C, reactive 8h, and naturally fell to room temperature after the reaction is stopped; the foam nickel is removed, and the surface deposit is removed, and the surface deposit is removed, and 12 h is dried in vacuo at 60 ° C;
[0032] (3) Put the foam nickel after step (2) in N 2 After the atmosphere is raised to 300 ° C in the atmosphere to 300 ° C, it will be pushed into the tube furnace and heat it up to room temperature, so that BI-coni can be obtained. ...
Embodiment 2
[0045] (1) Cut 1 cm * 3 cm foam nickel, with dilute hydrochloric acid, ethanol, deionized water to clean each 5 minutes, spare;
[0046] (2) Take a clean beaker, add 25ml to ion water, weigh 0.043 g Na 2 Bio 4 · 2h 2 O, 0.42g CO (NO 3 ) 2 · 6h 2 O and 0.08G Ni (NO 3 ) 2 · 6h 2 O Pour in deionized water, weigh 0.24 g 2-methylimidazole into a metal salt mixture, ultrasonic dissolution, pour in a 50 mL reaction kettle; the foam nickel after pre-treatment after step (1) is added to the reaction In the kettle, the oven is placed to 110 ° C, the reaction is 10 h, and after the reaction is stopped to room temperature; the foam nickel is removed, and the surface deposit is placed in the deionized water, and the surface deposit is removed, and 12 h is dried in vacuo at 60 ° C;
[0047] (3) Put the foam nickel after step (2) in N 2 After the atmosphere, it is raised from 10 ° C / min to 200 ° C, and the crucible of 150 mg of sulfur will be pushed into the tube furnace and heat it up to room...
Embodiment 3
[0050] (1) Cut 1 cm * 3 cm foam nickel, with dilute hydrochloric acid, ethanol, deionized water to clean each 5 minutes, spare;
[0051] (2) Take a clean beaker, add 25ml to ion water, weigh 0.06 g of NA 2 Bio 4 · 2h 2 O, 0.36g CO (NO 3 ) 2 · 6h 2 O and 0.08G Ni (NO 3 ) 2 · 6h 2 O Pour in deionized water, weigh 0.24 g 2-methylimidazole into a metal salt mixture, ultrasonic dissolution, pour in a 50 mL reaction kettle; the foam nickel after pre-treatment after step (1) is added to the reaction In the kettle, the oven is placed to 180 ° C, the reaction is 6 h, and after the reaction is stopped to room temperature; remove the foam nickel, placed in the deionized water for 30 seconds, remove the surface deposit, and dry at 60 ° C for 12 h;
[0052] (3) Put the foam nickel after step (2) in N 2 After the atmosphere is 10 ° C / min, it will be pushed into the tube furnace and heat it up to room temperature, which is reduced to room temperature to heat up to room temperature. 2 S 4 / NF,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com