Method for synergistically and deeply removing oxygen in metal titanium by super-oxyphilic metal and calcium
A metal titanium and metal technology, which is applied in the field of super-oxophilic metal-calcium synergistically deep removal of oxygen from metal titanium, can solve problems such as easy increase of calcium oxide activity and easy fluctuation of deoxidation effect, and the method is simple and easy to achieve. good removal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
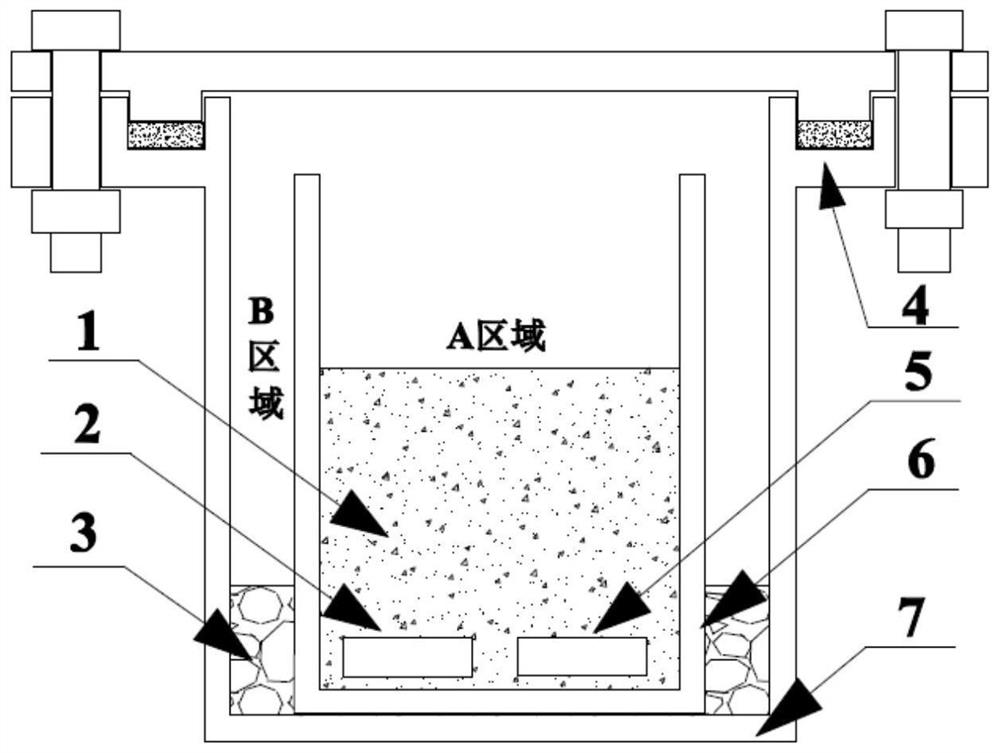
[0037] Get metal titanium band 15g, initial oxygen content 1330ppm, press (CaCl 2 ): m(Ti)=5:1 Add 75g of anhydrous calcium chloride (dehydrated at 500°C for 24 hours before use), add 10g of industrial calcium chips (at 900°C, the saturation solubility of calcium in anhydrous calcium chloride molten salt 2 times), add high-purity sponge yttrium 7.5g (50% of titanium raw material quality), add potassium chloride 22g (30% of anhydrous calcium chloride quality), metal titanium strip, anhydrous calcium chloride, chloride Potassium and high-purity sponge yttrium are filled in the titanium crucible, and industrial calcium chips are filled in the gap between the titanium crucible and the stainless steel crucible, and then the stainless steel crucible is sealed with a high-temperature sealing gasket. figure 1 shown.
[0038] Put the sealed crucible into a vacuum tank heated by a resistance furnace, cover the furnace cover, vacuumize to 0.04Pa, and then wash it with argon. Under the ...
Embodiment 2
[0040] Get metal titanium block 17g, initial oxygen content 1610ppm, press (CaCl 2 ): m(Ti)=8:1 Add 136g of anhydrous calcium chloride (dehydrated at 500°C for 24h before use), add 8g of distilled calcium chips (according to the saturation solubility of calcium in anhydrous calcium chloride molten salt at 800°C 1.5 times calculation), add high-purity yttrium block 5g (30% of titanium raw material quality), add potassium chloride 81g (60% of anhydrous calcium chloride quality), add metal titanium block, anhydrous calcium chloride, chloride Potassium and high-purity yttrium blocks are filled in the titanium crucible, and the distilled calcium chips are filled in the gap between the titanium crucible and the stainless steel crucible, and then the stainless steel crucible is sealed with a high-temperature sealing gasket. figure 1 shown.
[0041] Put the sealed crucible into a vacuum tank heated by a resistance furnace, cover the furnace cover, vacuumize to 0.4 Pa, and then wash w...
Embodiment 3
[0043] Get metal titanium rod 32g, initial oxygen content 2100ppm, press (CaCl 2 ): m(Ti)=20:1 Add 640g of anhydrous calcium chloride (dehydrated at 500°C for 24 hours before use), add 20.48g of high-purity calcium chips (at 1000°C, calcium saturated in anhydrous calcium chloride molten salt 0.5 times of solubility), add high-purity erbium plate 0.6g (2% of titanium raw material quality), do not add potassium chloride, metal titanium rod, anhydrous calcium chloride, high-purity erbium plate are filled in the titanium crucible, Fill the gap between the titanium crucible and the stainless steel crucible with high-purity calcium chips, and then seal the stainless steel crucible with a high-temperature sealing gasket. figure 1 shown.
[0044] Put the sealed crucible into a vacuum tank heated by a resistance furnace, cover the furnace cover, vacuumize to 0.1Pa, and then wash with argon. Under the protection of argon, heat up to 1000°C at a heating rate of 5°C / min, and keep at 100...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com