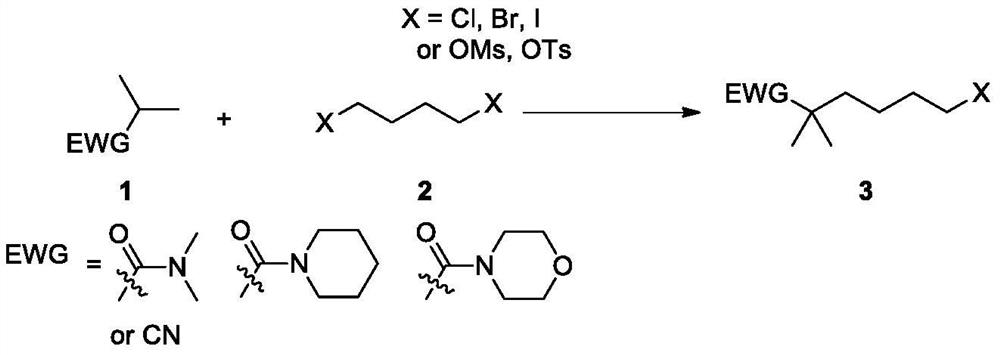
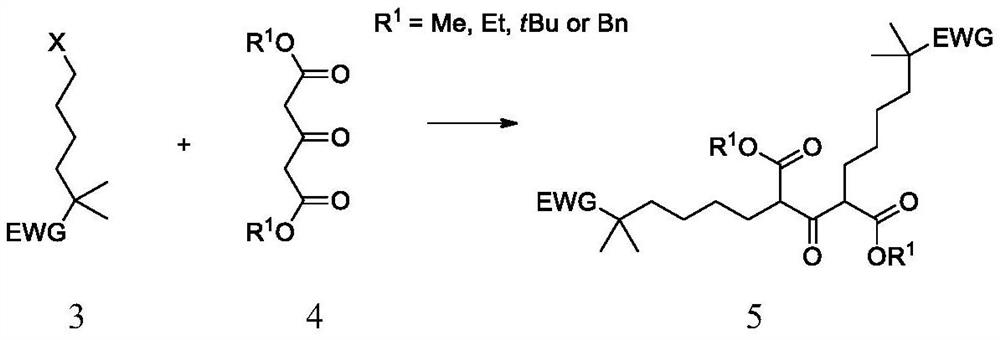
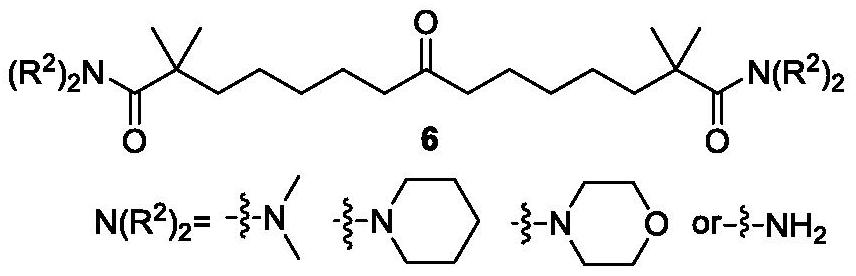
Synthetic method of bempedoic acid and intermediate thereof
A synthesis method and intermediate technology, which are applied in the field of chemical synthesis of raw materials, can solve the problems of difficult purification of final products, high cost of scaled production, and many side reactions in double alkylation reactions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070]
[0071] Add compound 1a (11.52g, 100mmol) and 57mL tetrahydrofuran into a three-necked flask, stir to dissolve and cool down to an internal temperature of about -70~-65°C, slowly add n-butyllithium solution (1M, 105mL, 105mmol) dropwise, and add After the completion, keep stirring for 0.5 hours, then add dropwise the THF solution of 2a (21.59g, 100mmol, dissolved in 57mL THF), stir evenly and then naturally heat up to 20-30°C for 2-3 hours. Dilute hydrochloric acid (5%, 115mL) was added at the end of the reaction, ethyl acetate (115mL) was added to extract three times, the combined organic phase was washed twice with saturated brine, dried, filtered, concentrated, and distilled under reduced pressure to obtain compound 3a (19.64g, 78.6 %). MS(ESI)m / z=250.1[M+H] +
[0072] In Example 1, tetrahydrofuran can be replaced by N,N-dimethylformamide, N,N-dimethylacetamide, N-methylpyrrolidone, acetonitrile or 2-methyltetrahydrofuran; n-butyllithium can be replaced by LDA,...
Embodiment 2
[0075]
[0076] Add compound 1b (15.72g, 100mmol) and 78mL tetrahydrofuran into a three-neck flask, stir to dissolve and cool down to an internal temperature of about -70~-65°C, slowly drop in LDA solution (1M, 105mL, 105mmol), keep warm after the addition is complete Stir for 0.5 hour, then drop into the THF solution of 2a (21.59g, 100mmol, dissolved in 77mL THF), stir well, then naturally heat up to 20-30°C and react for 2-3 hours. Dilute hydrochloric acid (5%, 156mL) was added at the end of the reaction, ethyl acetate (156mL) was added to extract 3 times, the combined organic phase was washed 2 times with saturated brine, dried, filtered, concentrated, and distilled under reduced pressure to obtain compound 3b (23.00g, 78.7 %). MS(ESI)m / z=292.0[M+H] +
[0077] In Example 2, tetrahydrofuran can be replaced by N,N-dimethylformamide, N,N-dimethylacetamide, N-methylpyrrolidone, acetonitrile or 2-methyltetrahydrofuran; LDA can be replaced by n-butyllithium, tert-butyl Sodi...
Embodiment 3
[0080]
[0081] Add compound 1c (15.52g, 100mmol) and 77mL tetrahydrofuran into a three-neck flask, stir to dissolve and cool at low temperature to an internal temperature of about -15~-10°C, slowly add LiHMDS (1M toluene solution, 105mL), keep stirring for 0.5 hours after the addition is complete , and then drop into the tetrahydrofuran solution of 2a (21.59g, 100mmol, dissolved in 77mL tetrahydrofuran), stir evenly and then naturally heat up to 0-5°C to react for 2-3 hours. After the reaction, dilute hydrochloric acid (5%, 155 mL) was added, ethyl acetate (154 mL) was added to extract 3 times, the combined organic phases were washed with saturated brine twice, dried, filtered, concentrated, and distilled under reduced pressure to obtain compound 3c (23.02 g, 79.3 %).
[0082] MS(ESI)m / z=290.0[M+H] +
[0083] In Example 3, tetrahydrofuran can be replaced by N,N-dimethylformamide, N,N-dimethylacetamide, N-methylpyrrolidone, acetonitrile or 2-methyltetrahydrofuran; LiHMDS ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com