Preparation method and application of ammonium perchlorate thermal decomposition catalytic material
A catalytic material, ammonium perchlorate technology, applied in chemical instruments and methods, non-explosive/non-thermal agent components, physical/chemical process catalysts, etc., can solve the problems of poor catalytic performance of AP thermal decomposition, etc., to increase catalytic activity site, good application prospects, and the effect of increasing the specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
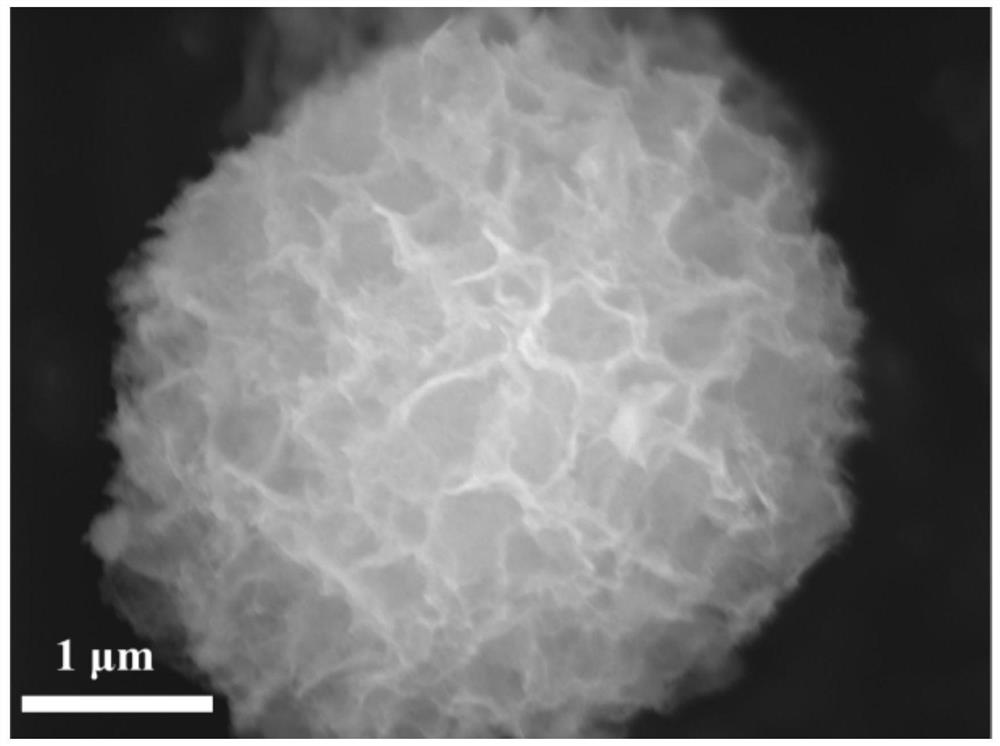
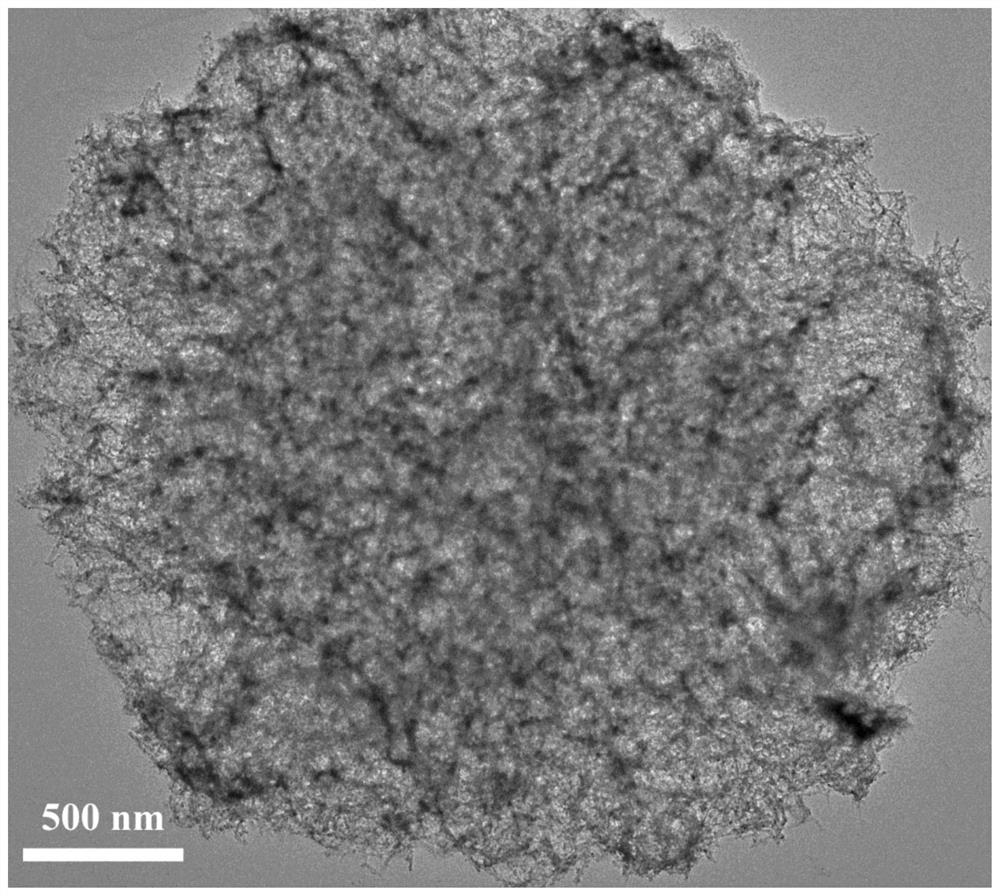
[0023] 4mmol Co(NO 3 ) 2 ·6H 2 O, 2mmol Cu(NO 3 ) 2 ·6H 2 O and 12 mmol urea were dissolved in 50 mL of polyethylene glycol 200 (PEG-200) to form a transparent pink solution, then the above mixture solution was transferred to a stainless steel reaction kettle containing a polytetrafluoroethylene liner, and reacted at 180 °C 12 hours. After natural cooling to room temperature, the resulting solid was filtered, washed thoroughly with deionized water and ethanol three times, and dried in an oven at 60 °C to obtain nanoflower-like CuCo 2 o 4 product.
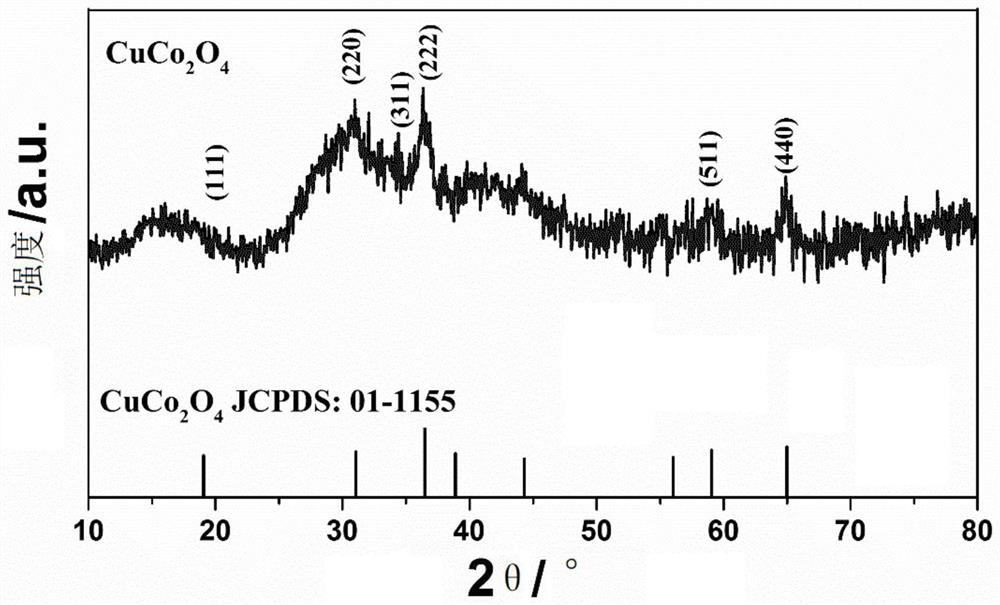
[0024] figure 1 For the nano-flower-like structure CuCo prepared in Example 1 2 o 4 XRD phase analysis of materials, from figure 1 It can be seen that CuCo 2 o 4 All the diffraction peaks and corresponding diffraction crystal planes in the sample are consistent with the spinel structure CuCo 2 o 4 The standard data (JCPDS Card No.01-1155) is consistent. The spectrum does not show any other impurity peaks, indicating ...
Embodiment 2
[0027] 4mmol Co(NO 3 ) 2 ·6H 2 O, 2mmol Cu(NO 3 ) 2 ·6H 2 O and 12 mmol of urea were dissolved in 50 mL of polyethylene glycol 200 (PEG-200) to form a transparent pink solution, then the above mixture solution was transferred to a stainless steel reaction kettle containing a polytetrafluoroethylene liner, and reacted at 200 °C 12 hours. After natural cooling to room temperature, the resulting solid was filtered, washed thoroughly with deionized water and ethanol three times, and dried in an oven at 60 °C to obtain nanoflower-like CuCo 2 o 4 product.
Embodiment 3
[0029] 4mmol Co(NO 3 ) 2 ·6H 2 O, 2mmol Cu(NO 3 ) 2 ·6H2 O and 12 mmol of urea were dissolved in 50 mL of polyethylene glycol 200 (PEG-200) to form a transparent pink solution, and then the above mixture solution was transferred to a stainless steel reactor containing a polytetrafluoroethylene liner and reacted at 160 °C 12 hours. After natural cooling to room temperature, the resulting solid was filtered, washed thoroughly with deionized water and ethanol three times, and dried in an oven at 60 °C to obtain nanoflower-like CuCo 2 o 4 product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com