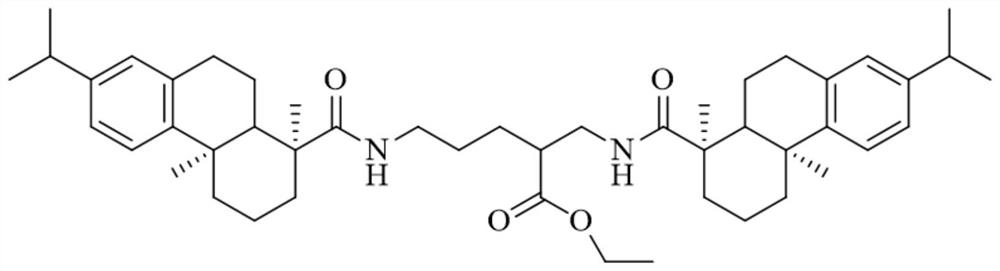
Rosin-based micromolecular organic gel and cyclo-hexane gel formed by same
A technology of cyclohexane gel and organogel, which is applied in the field of cyclohexane gel, can solve the problems of no preparation method of rosin-based organogel, and achieve the effects of promoting development, rich resources, and unique chemical structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Purification of dehydroabietic acid: put 50g of disproportionated rosin, 5mL of ethanol and 250mL of petroleum ether into a clean 1000mL beaker, heat and stir to dissolve it completely, add ethanolamine (7.13 g, 0.12mol), and after fully stirring for 20 minutes, a solid precipitated out, cooled naturally to room temperature, and filtered under reduced pressure. The obtained solid was recrystallized three times with ethanol / water (1:1 by volume) to obtain relatively pure ethanolamine salt of dehydroabietic acid. Dissolve the above-mentioned ethanolamine salt with 100mL ethanol / water (volume ratio 1:1) solution under heating, and acidify with acetic acid (10.5g, 0.18mol). During the process, a white solid precipitates, then add 10mL water to make it fully precipitated, and cool to Suction filtration under reduced pressure at room temperature, and vacuum drying to obtain pure white dehydroabietic acid.
[0031] (2) Synthesis of rosin-based amphiphile: dissolve ethyl l...
Embodiment 2
[0034] [Example 2] Determination of the gelation behavior of rosin-based amphiphiles.
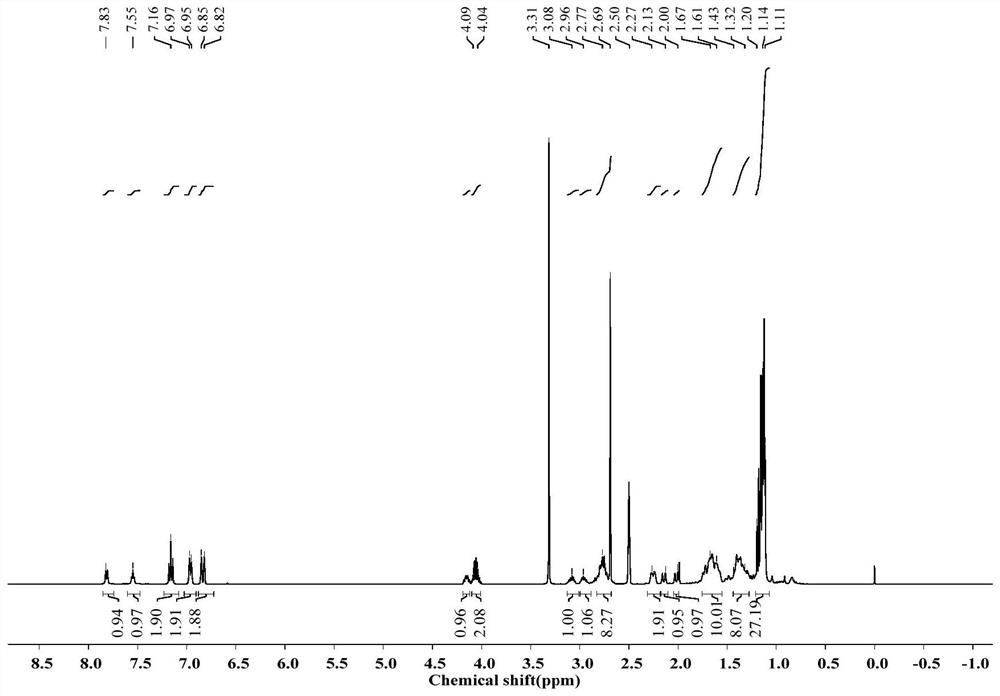
[0035] Weigh 0.025g of rosin-based amphiphile and 1mL of different solvents (the concentration of the corresponding solution is recorded as 2.5%, w / v) and put them in a glass bottle, seal it, heat it until it is completely dissolved, and observe it upside down after cooling at room temperature. The results are shown in Table 1. The rosin-based amphiphile exhibited good gelling ability in the solvents cyclohexane and TEOS. image 3 A picture of a cyclohexane gel formed between a rosin-based amphiphile and cyclohexane.
[0036] The gel behavior (2.5%, w / v) of table 1 compound 1 in various organic solvents
[0037]
[0038] Note: [a] G = stable gel S = dissolved I = insoluble
[0039] Determination of the microstructure of rosin-based amphiphiles:
[0040] After freeze-drying the wet gel formed by the above 0.025g rosin-based amphiphile and 1mL cyclohexane, it was coated on the conducti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Resonance frequency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com