Synthesis method of 2-amino-3, 5-dichloro-N-methylbenzamide
A technology of toluamide and synthesis method, which is applied in the field of synthesis of 2-amino-3,5-dichloro-N-methylbenzamide, can solve the problem of low chlorination reaction activity and increased dichlorination Hydantoin, low product purity and other problems, to achieve the effect of good chlorination reaction, good solubility and high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
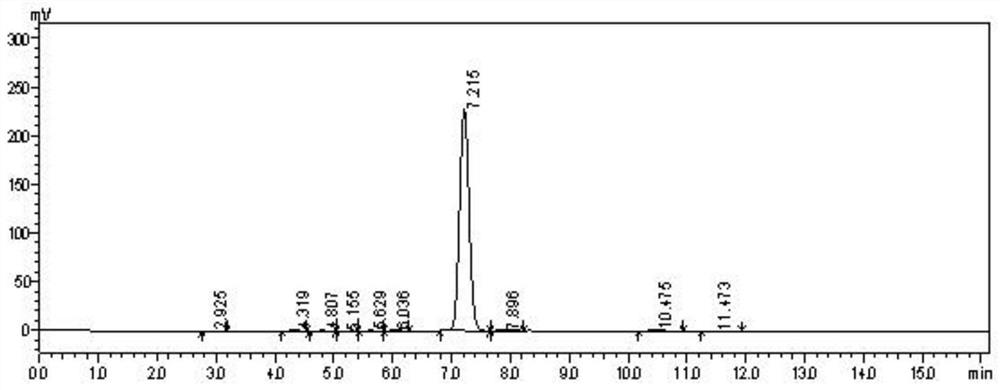
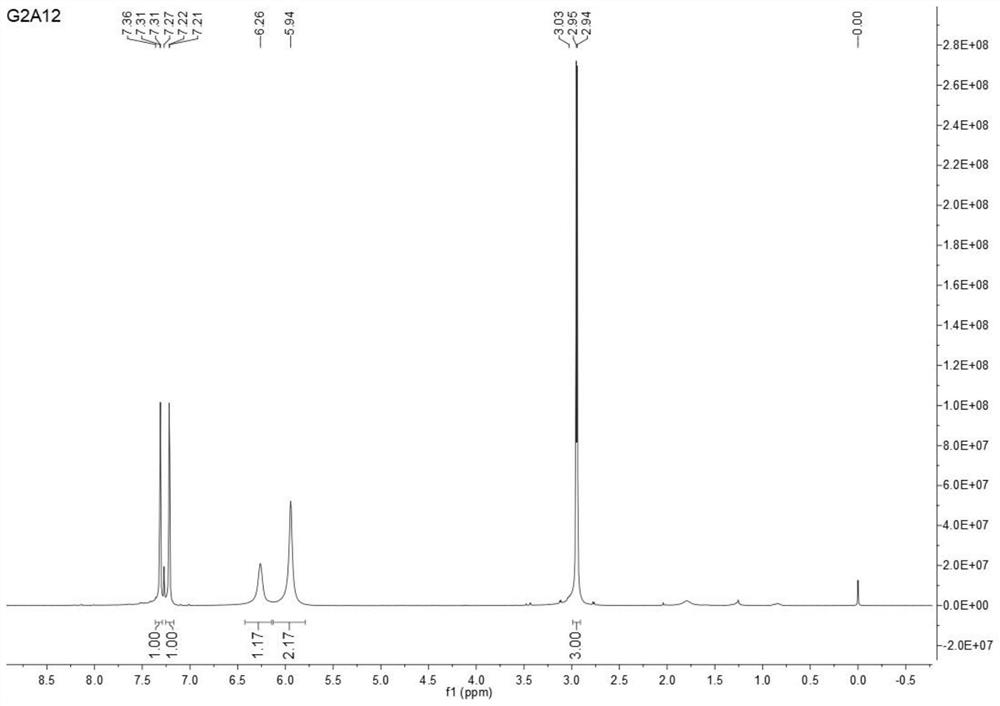
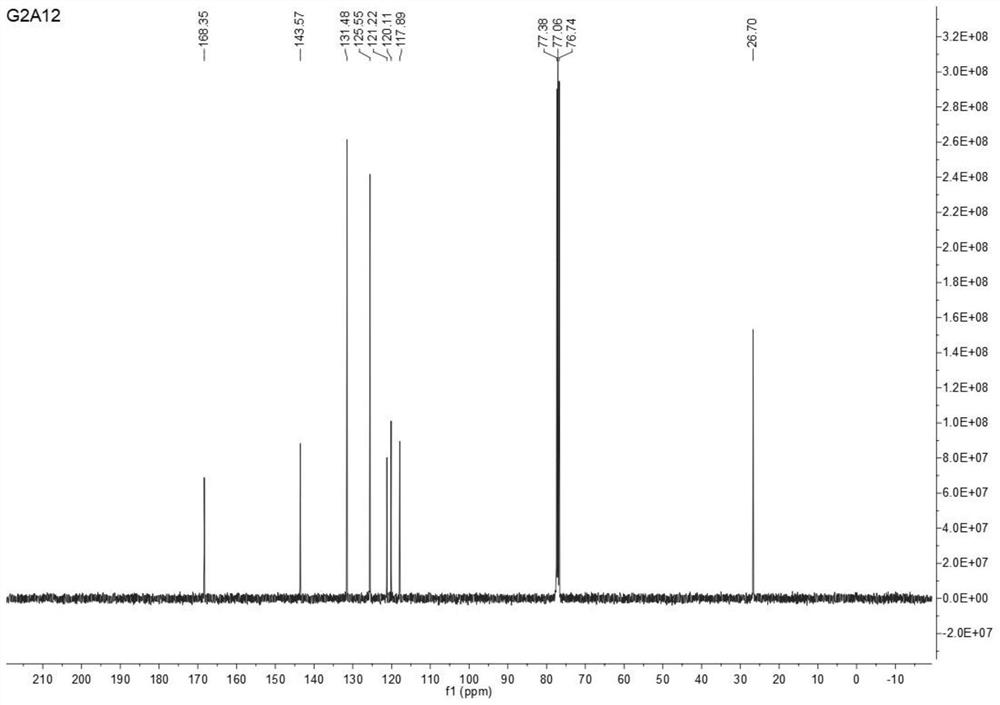
Image
Examples
preparation example Construction
[0035] The invention discloses a synthesis method of 2-amino-3,5-dichloro-N-methylbenzamide. The synthesis method is a one-pot method, comprising the following steps:
[0036] 1) Add isatoic anhydride to solvent A, add methylamine solution dropwise to react, the reaction temperature can be controlled at about 10°C to 50°C, and the effect is best when the temperature is 30°C. After the reaction, add water and stir evenly Set the layers, extract the organic phase, the organic phase is 2-amino-N-methylbenzamide;
[0037] 2) Slowly add trichloroisocyanuric acid to the organic phase extracted in step 1) for reaction. The reaction temperature can be controlled at about 30°C-80°C, and the effect is best when the temperature is 40°C-50°C. After the reaction, Remove the solvent, add water, and adjust the pH to 8-13. You can use sodium hydroxide, sodium carbonate and other reagents to adjust the pH value. After adjusting the pH, perform suction filtration. After suction filtration, a wh...
Embodiment 1
[0048] At room temperature, in a 500ml round bottom flask, add 50g of isatoic anhydride and 250ml of ethyl acetate, control the reaction temperature not to exceed 30°C, add 37.3g of 25% methylamine solution dropwise, after the dropwise addition, keep warm at 30°C for reaction After 4 hours, the reaction was monitored by liquid chromatography, and isatoic anhydride disappeared. Add 100ml of water, stir evenly, and let stand to separate layers. The upper layer is an ethyl acetate organic phase, and the lower layer is an aqueous phase. The liquid was separated and the aqueous phase was discarded.
[0049] Add 51.3g of trichloroisocyanuric acid directly to the organic phase in batches, and control the temperature not to exceed 40°C when feeding. After feeding, keep warm at 50°C for reaction, and use HPLC to monitor the product 2-amino-N-formazan in the previous step. After methyl benzamide and monochlorinated 2-amino-N-methylbenzamide disappear, add 10g of sodium sulfite to quen...
Embodiment 2
[0052] At room temperature, in a 500ml round bottom flask, add 50g of isatoic anhydride and 300ml of isopropyl acetate, control the reaction temperature not to exceed 30°C, add 37.3g of 25% methylamine aqueous solution dropwise, after the dropwise addition, keep warm at 30°C After 4 hours of reaction, the reaction was monitored by liquid chromatography, and isatoic anhydride disappeared. Add 100ml of water, stir evenly, and let stand to separate layers. The upper layer is the organic phase of isopropyl acetate, and the lower layer is the water phase, and the liquid is separated, and the water phase is discarded.
[0053] Add 51.3g of trichloroisocyanuric acid directly to the organic phase in batches, and control the temperature not to exceed 40°C when feeding. After feeding, keep warm at 50°C for reaction, and use HPLC to monitor the product 2-amino-N-formazan in the previous step. After the disappearance of phenylbenzamide and monochlorinated 2-amino-N-methylbenzamide. Add ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com