Preparation method of sulfonated cellulose
A technology of cellulose and dialdehyde cellulose, which is applied in the field of sulfonated cellulose preparation, can solve the problems of lower yield, large consumption of deionized water, time-consuming and labor-consuming, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] A method for preparing sulfonated cellulose, the preparation method comprising the step of preparing sulfonated cellulose through an addition reaction between dialdehyde cellulose and a sulfonating agent, wherein the reaction medium of the addition reaction contains ethanol.
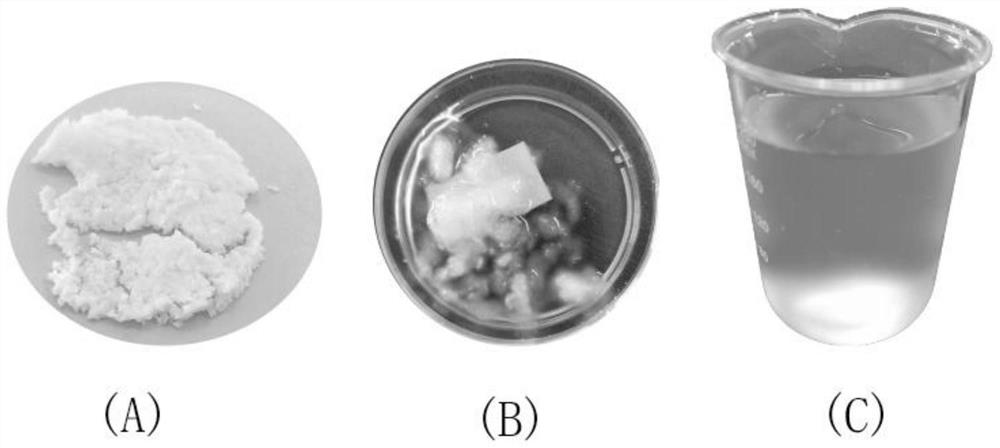
[0031] At present, in most cases, when preparing sulfonated cellulose through the addition reaction of dialdehyde cellulose and sulfonating agent, the selected reaction medium is water. However, the inventors have found that when water is used as the reaction medium, if the aldehyde group content of dialdehyde cellulose is low, the correspondingly prepared sulfonated cellulose does not have high hydrophilicity. At this time, the obtained sulfonated cellulose can be compared It is easily separated from the reaction system by conventional separation methods (such as filtration, etc.), and it is also relatively easy to purify for subsequent use. However, if the aldehyde group content of dialdehyde ce...
Embodiment 1
[0056] This example provides a method for preparing sulfonated cellulose, the preparation process is shown in figure 1 , including the following steps:
[0057] Step 1, preparation of dialdehyde cellulose.
[0058] (1) Weigh 4g microcrystalline cellulose into a 250ml Erlenmeyer flask, add 200ml distilled water, 5.28g NaIO 4 (molar ratio NaIO 4 :AGU=1:1) and 3.364g sodium chloride (molar ratio NaCl:AGU=7:3), and cover the Erlenmeyer flask with aluminum foil to prevent photocatalytic decomposition of periodate.
[0059] (2) Stir the reaction at 50° C. in a water bath for 3 hours.
[0060] (3) After the reaction is over, add 3ml of ethylene glycol and continue the reaction for 10 minutes.
[0061] (4) Filtrate and wash with deionized water for several times until the conductivity is less than 50 μS / cm, wash with ethanol twice, and then vacuum-dry to prepare absolute-dry dialdehyde cellulose.
[0062] Step 2, preparation of sulfonated cellulose.
[0063] Pipette 70ml of etha...
Embodiment 2
[0088] This embodiment is a modification of embodiment 1. Compared with embodiment 1, the only difference is that the ratio (volume ratio) of ethanol to water in step 2 is 10:0. Specifically, the steps of this embodiment include:
[0089] Step 1, preparation of dialdehyde cellulose.
[0090] (1) Weigh 4g microcrystalline cellulose into a 250ml Erlenmeyer flask, add 200ml distilled water and 5.28g NaIO 4 (molar ratio NaIO 4 :AGU=1:1) and 3.364g sodium chloride (molar ratio NaCl:AGU=7:3), and cover the Erlenmeyer flask with aluminum foil to prevent photocatalytic decomposition of periodate.
[0091] (2) Stir the reaction at 50° C. in a water bath for 3 hours.
[0092] (3) After the reaction is over, add 3ml of ethylene glycol and continue the reaction for 10 minutes.
[0093] (4) Filtrate and wash with deionized water for several times until the conductivity is less than 50 μS / cm, wash with ethanol twice, and then vacuum-dry to prepare absolute-dry dialdehyde cellulose.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
| Surface charge density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com