Bovine pasteurella multocida inactivated vaccine and preparation method thereof
A technology of Pasteurella and inactivated vaccines, which is applied in the field of inactivated vaccines against Pasteurella multocida and its preparation, which can solve the problems of low number of Pasteurella cultured viable bacteria, poor product potency, and toxic and side effects and other problems, to achieve the effect of increasing the number of viable bacteria, improving quality, and reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation method of bovine pasteurellosis multocida inactivated vaccine in the present embodiment may further comprise the steps:
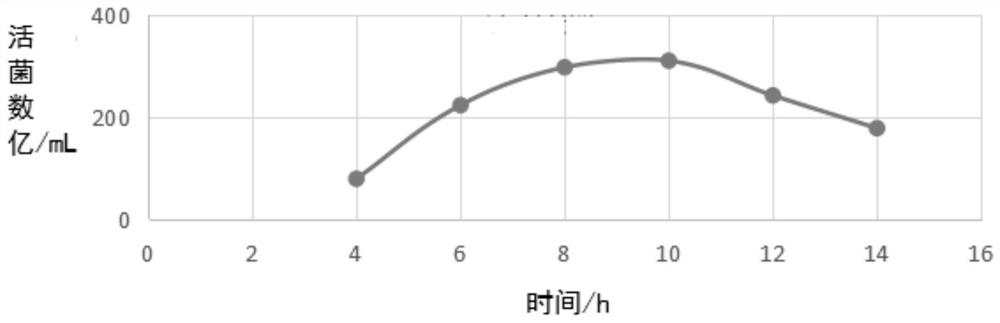
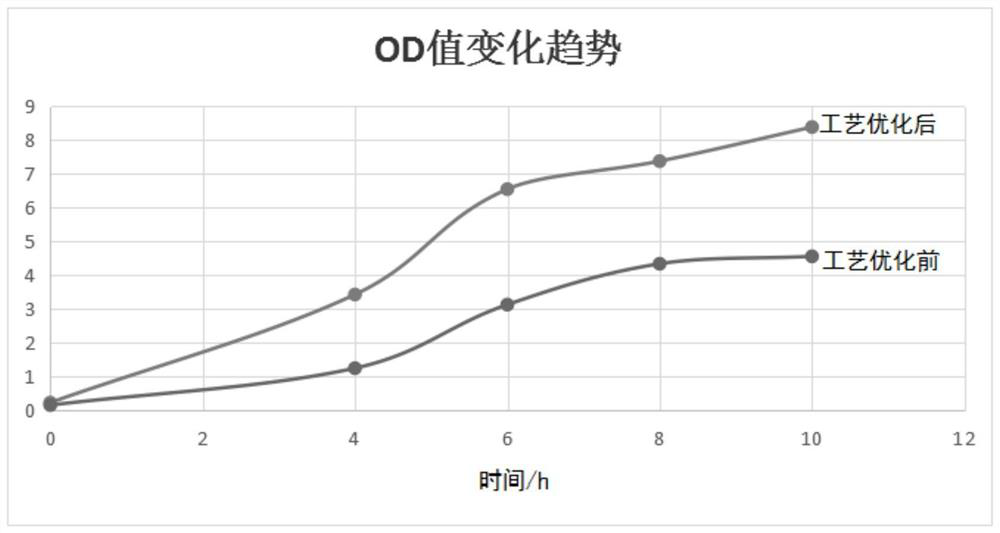
[0032] 1) Primary seed preparation: Inoculate 1% of each freeze-dried strain into Martin broth medium containing 0.1% lysed whole blood and 4% healthy horse serum, and culture on a shaker at 150rpm at 37°C After 12 hours, the culture was streaked on a modified Martin agar plate containing 0.5% lysed whole blood and 4% healthy horse serum, cultivated for 12 hours at 37°C, and more than 5 smooth typical colonies were selected and inoculated on A number of blood slant were cultivated for 24 hours at 37°C to obtain first-grade seeds;
[0033] 2) Secondary seed preparation: inoculate the primary seeds of each strain in the Martin Broth medium containing 0.1% lysed whole blood and 4% healthy horse serum according to the inoculum size of 1%, and culture them on a shaking table at 37°C for 12 Hours, microscopic examination and pure inspectio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com