Metal material and preparation method thereof as well as steel structural member
A technology of metal materials and steel structural parts, applied in the field of metal materials and their preparation, can solve the problems of electrochemical corrosion, short corrosion resistance life, large amount of electrochemical corrosion, etc., and achieve good affinity and adhesion, high strength and toughness Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
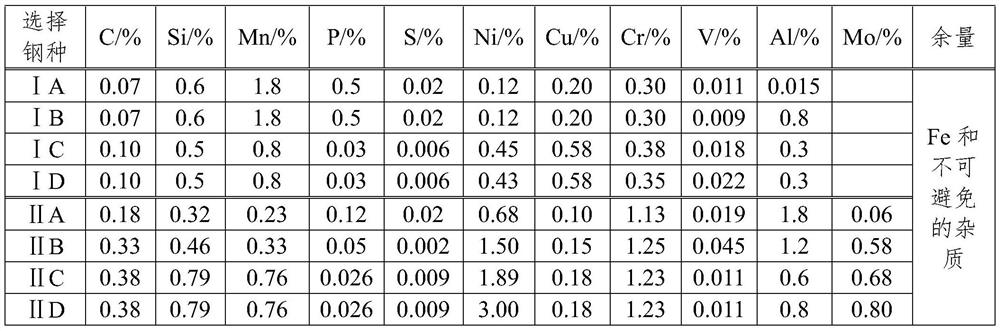
[0060] Design and calculation of electrochemical galvanic corrosion amount of bolts made of a metal material in this embodiment, wherein the test piece is an M8 screw (surface zinc alloy) made of a metal material of group II C, which is combined with a 5mm aluminum plate The threaded connection forms a threaded pair. In the design of the thread pair, the service life of the thread height K after galvanic corrosion Cl should be satisfied, K l Take 50 years (building design life is 50 years, 70 years, 100 years), η takes 1.2, γ q Take 1.4, M8 thread height takes 0.6mm, the calculation is as follows:
[0061] K Cl =K CO -K C ·K l ·Δ / η
[0062] min=K Cl / K CO ≥0.9
[0063] K Cl =0.6mm-[0.00000096×50×2 / 1.2]
[0064] =0.6mm-0.00008
[0065] =0.59992mm
[0066] min=0.59992÷0.6
[0067] =0.9999≥0.9, so the thread height meets the design requirements!
[0068] In the formula:
[0069] K Cl ——Thread design age; thread height after galvanic corrosion;
[0070] K CO ——...
example 2
[0087] Design calculation of electrochemical galvanic corrosion amount of 304 stainless steel screws after surface passivation treatment using the formula in Example 1, K l Take 50 years, η takes 1.2, γ q Take 1.4, M8 thread height takes 0.6mm, and carry out design checking:
[0088] K Cl =K CO -K C ·K l ·Δ / η
[0089] min=K Cl / K CO ≥0.9
[0090] K Cl =0.6mm-[0.002×50×2×1.2]
[0091] =0.6mm-0.24mm
[0092] =0.36mm
[0093] min=0.36÷0.6≥0.9
[0094] =0.6<0.9, so the thread height does not meet the design requirements!
[0095] Substitute into the above formula:
[0096]
example 3
[0098] Design calculation of electrochemical galvanic corrosion amount of 316 stainless steel screws after surface passivation treatment using the formula in Example 1, K l Take 50 years, η takes 1.2, γ q Take 1.4, M8 thread height takes 0.6mm, and carry out design checking:
[0099] K Cl = K CO -K C ·K l ·Δ / η
[0100] min=K Cl / K CO ≥0.9
[0101] K Cl =0.6mm-[0.0027×50×2×1.2]
[0102] =0.6mm-0.324mm
[0103] =0.276mm
[0104] min=0.276÷0.6≥0.9
[0105] =0.46<0.9, so the thread height does not meet the design requirements!
[0106] Substitute into the above formula:
[0107]
[0108] To sum up, the screws made of metal materials in group II C, after the surface is infiltrated with zinc alloy, have good performance in preventing electrochemical corrosion and have a longer service life.
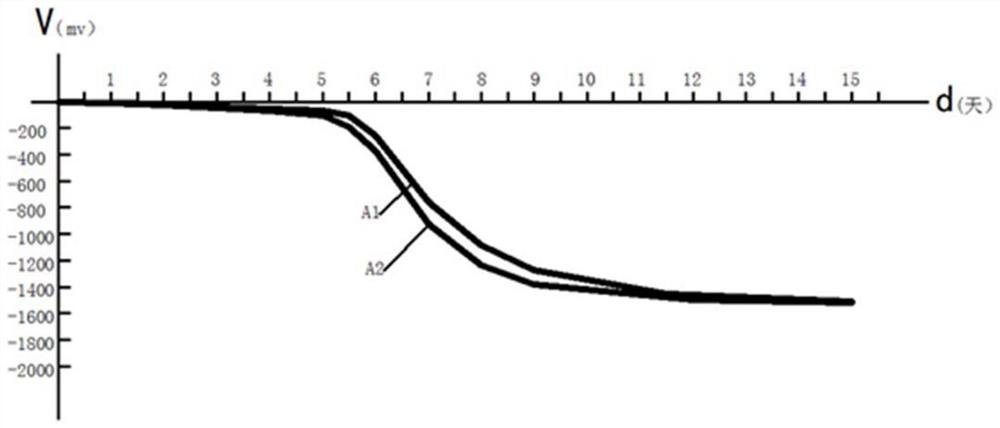
[0109] In this embodiment, the electrochemical corrosion performance of the screw made of the metal material of this embodiment can also be evaluated by using the zero-resistanc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com