Synthesis method of 1,3-benzodiazepine compounds and antitumor activity
A technology for benzodiazepines and a synthesis method, which is applied in the directions of organic chemistry, drug combination, antitumor drugs, etc., can solve the problems of single product structure, difficult to obtain raw materials, low atom economy and the like, and achieves simple synthesis process and raw materials. Inexpensive and readily available raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
[0029] Add compound 1a, solvent, catalyst, additive and compound 2a to a 15mL reaction bottle in sequence, cover with a stopper and seal it, place it in an oil bath to heat up and stir for reaction. After the reaction was completed, it was cooled to room temperature, filtered with suction, spin-dried, and separated through a silica gel column (petroleum ether / ethyl acetate=300 / 1) to obtain product 3a as a yellow solid.
[0030] A series of results were obtained by changing reaction conditions such as catalyst, additive, organic solvent, equivalent ratio between reactants and reaction temperature, as shown in Table 1.
[0031] Synthesis of 3a under different conditions in table 1 a
[0032]
[0033]
Embodiment 2
[0035]
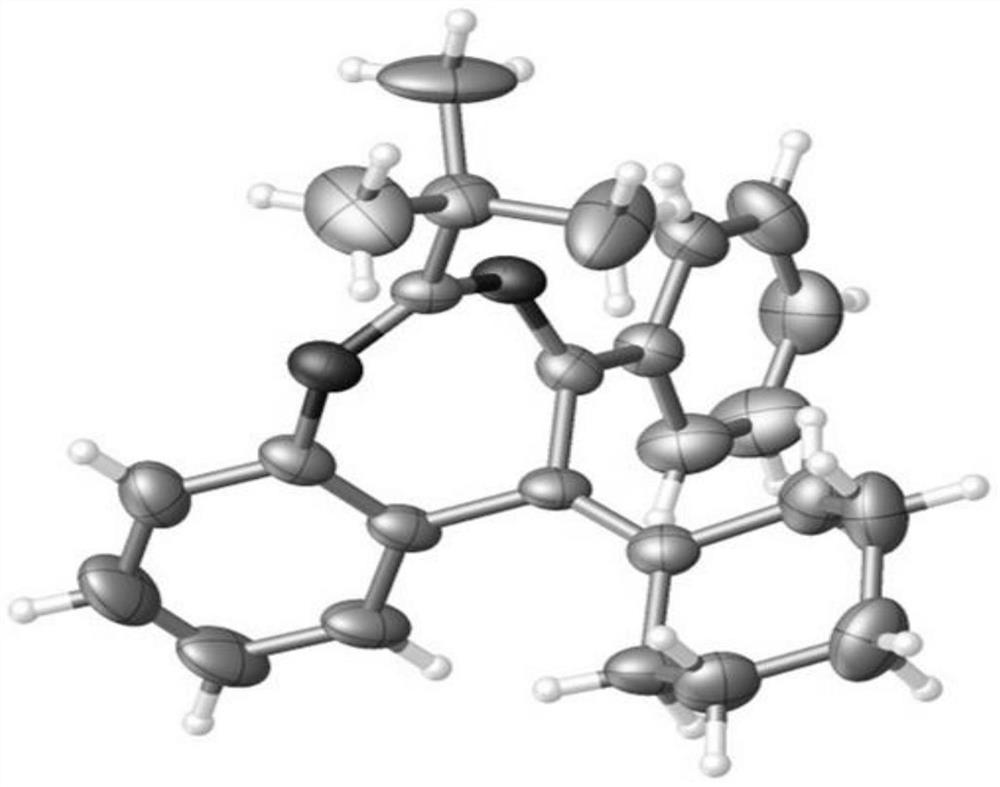
[0036] To a 15mL reaction flask, add 1a (53mg, 0.3mmol), methanol (1mL), dichloro(pentamethylcyclopentadienyl) rhodium (III) dimer (5.6mg, 0.009mmol), acetic acid Copper monohydrate (15mg, 0.075mmol) and compound 2a (72.8mg, 0.36mmol) were capped and sealed, and placed in an oil bath at 90°C and stirred for 5h. After the reaction, cooled to room temperature, suction filtered, spin-dried, and separated by silica gel column (petroleum ether / ethyl acetate=300 / 1) to obtain yellow solid product 3a (68.4 mg, 72%). The characterization data of this compound are: 1 H NMR (400MHz, CDCl 3 )δ1.27(s,9H),1.60(s,3H),1.68(s,3H),7.09-7.15(m,2H),7.21(td,J 1 =8.0Hz,J 2 =2.0Hz,1H),7.29-7.35(m,4H),7.93-7.95(m,2H). 13 C NMR (100MHz, CDCl 3 )δ19.8, 21.5, 28.4, 39.7, 125.7, 127.0, 127.7, 128.1, 128.7, 128.9, 129.9, 130.7, 131.4, 131.8, 135.7, 145.3, 165.1, 172.1. HRMS calcd for C 22 h 25 N 2 :317.2012[M+H] + ,found: 317.2012.
Embodiment 3
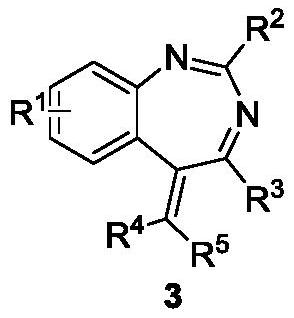
[0038] According to the method and steps of embodiment 2a,b , by changing reactant 1 and reactant 2, a series of 1,3-benzodiazepine compounds 3a-3z and 3aa-3qq were synthesized, the specific results are as follows:
[0039]
[0040]
[0041] a Reaction conditions: 1 (0.3mmol), 2 (0.36mmol), [RhCp*Cl 2 ] 2 (3mol%), Cu(OAc) 2 ·H 2 O (25mol%), MeOH (1mL), 90°C, 5h; b Separation yield; c With AgOAc (25mol%) and NaHCO 3 (0.3mmol) instead of Cu(OAc) 2 ·H 2 O (25mol%), 10h.
[0042] ________________________________________________________________
[0043] Representative product characterization data are as follows:
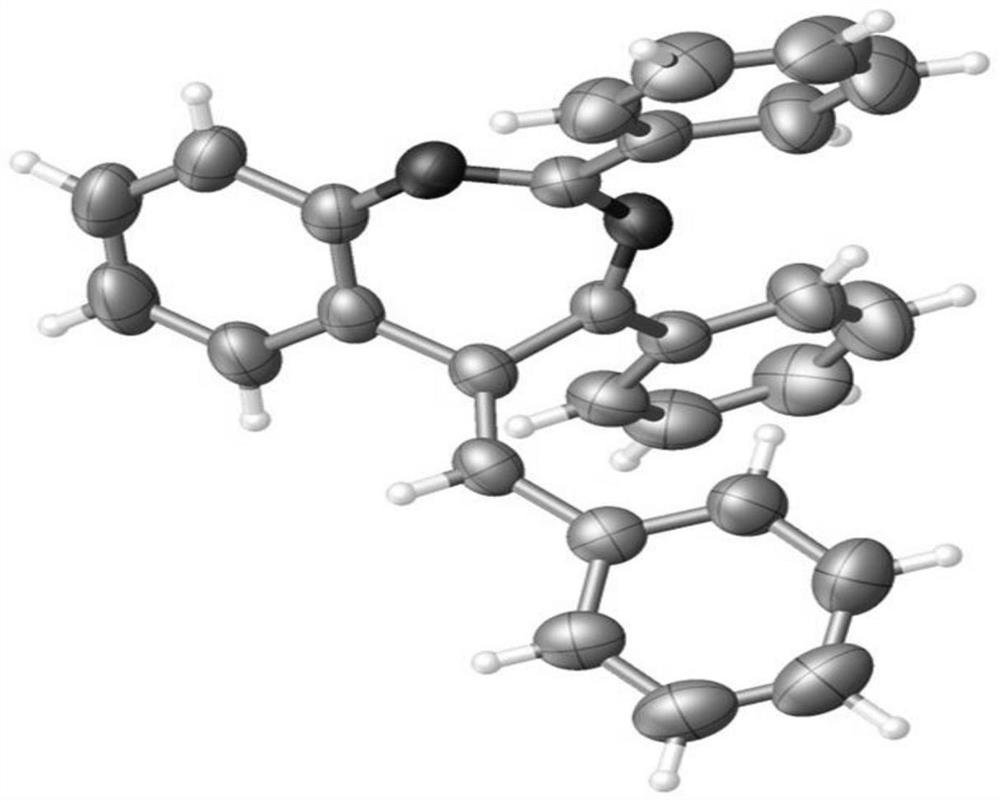
[0044] 2-(tert-Butyl)-7-methyl-4-phenyl-5-(propan-2-ylidene)-5H-benzo[d][1,3]diazepine(3b)
[0045] Yellow oil (66.4mg, 67%). 1 H NMR (400MHz, CDCl 3 )δ1.33(s,9H),1.66(s,3H),1.75(s,3H),2.33(s,3H),6.96(s,1H),7.09(dd,J 1 =8.4Hz,J 2 =1.6Hz,1H),7.29(d,J=8.0Hz,1H),7.38-7.43(m,3H),8.02(dd,J 1 =8.0Hz,J 2 =2.0Hz,2H). 13 C NMR (150MHz, CDCl 3 )δ19.8,21.1,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com