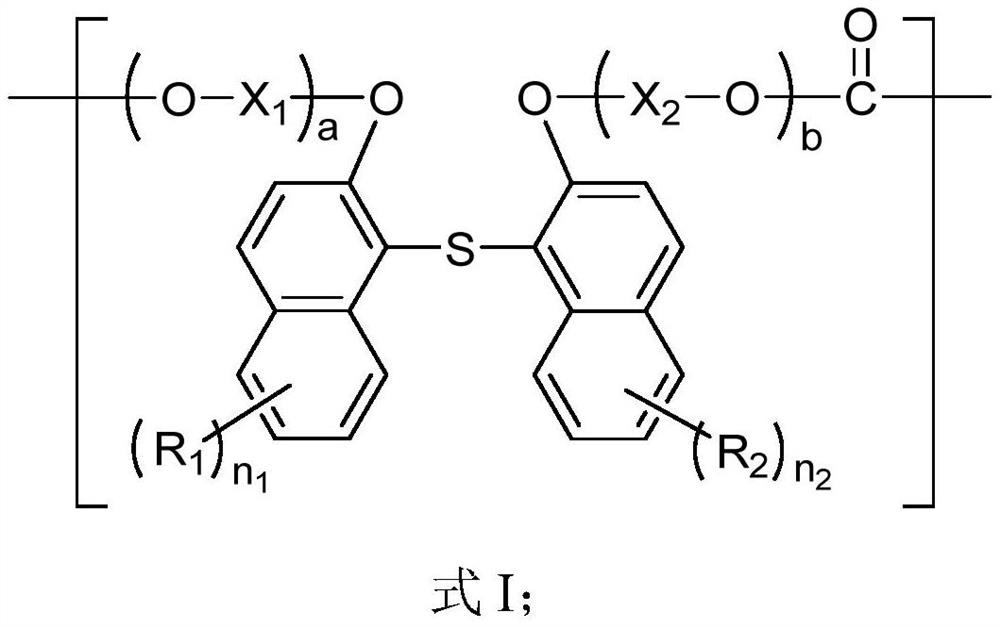
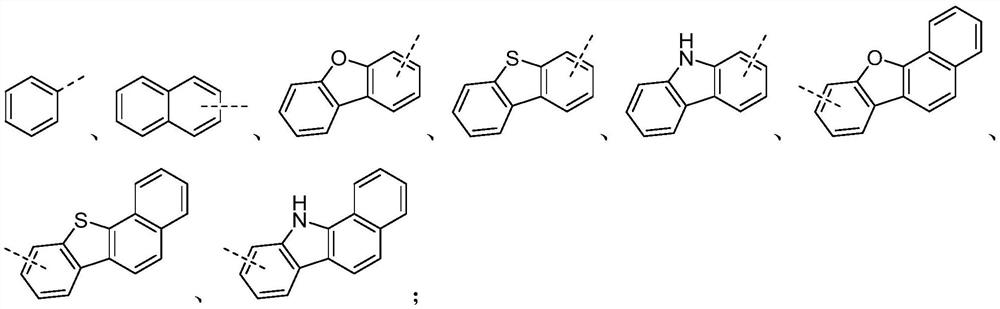
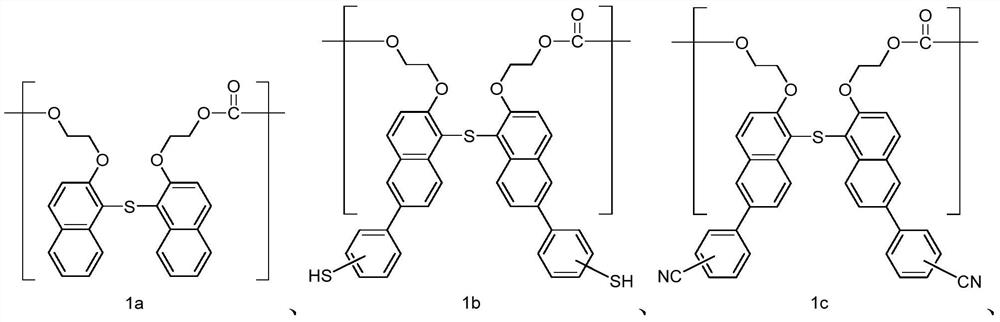
Polycarbonate and preparation method and application thereof
A polycarbonate, an independent technology, applied in the field of polycarbonate and its preparation, can solve the problems of complex lens composition, large chromatic aberration, etc., and achieve the effect of multi-selectivity, high glass transition temperature, and high refractive index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0160] 2,2-bis(2-hydroxyethoxy)-6,6-diphenyl-1,1-dithionaphthalene (BINSL-2EO)
[0161] The synthetic route is as follows:
[0162]
[0163] (1) In a four-necked flask equipped with a magnetic stirrer, a thermometer, a constant pressure dropping funnel and a condensing reflux tube, add 223g (1mol) of 6-bromo-2-naphthol (Wuhan Rongcan Biopharmaceutical) and toluene and Mixed solution 500mL of ethyl acetate (mass ratio of toluene to ethyl acetate is 3:2), stir to make it dissolve, under ice bath condition, slowly add sulfur dichloride 53g (0.52mol), control reaction system temperature at Around 15°C. After dripping, continue to react for 3h. Raise the temperature to 100°C, distill off most of the solvent, put it in the refrigerator to freeze for 1 hour, a solid precipitates out, filter, recrystallize with absolute ethanol, filter and dry to obtain 6,6-dibromo-1,1-dithionaphthalene phenol.
[0164] (2) In the reactor, 6,6-dibromo-1,1-dithionaphthalene 1mol and phenylboroni...
preparation example 2
[0168] 2,2-bis(2-hydroxyethoxy)-6,6-bis(naphthalene-1-yl)-1,1-dithionaphthalene (1DNBINSL-2EO)
[0169] The synthetic route is as follows:
[0170]
[0171] The difference between the specific preparation method and Preparation Example 1 is that the phenylboronic acid in step (2) is replaced with an equimolar amount of 1-naphthylboronic acid (Puyang Huicheng Technology); other reaction steps are the same as Preparation Example 1, The target product 1DNBINSL-2EO was obtained.
[0172] The NMR results of the target product are as follows: 1 H-NMR (400MHz, CDCl 3 )δ / ×10 -6 : 8.55(t, 2H), 8.42(t, 2H), 8.08-8.04(m, 6H), 7.95(t, 2H), 7.86(d, 2H), 7.61-7.55(m, 8H), 6.85(d , 2H), 4.43(t, 4H), 3.69(m, 4H), 3.65(m, 2H).
preparation example 3
[0174] 2,2-bis(2-hydroxyethoxy)-1,1-dithionaphthalene (BNSE)
[0175] The synthetic route is as follows:
[0176]
[0177] (1) In a four-necked flask equipped with a magnetic stirrer, a thermometer, a constant pressure dropping funnel and a condensing reflux tube, add 144g (1mol) of 2-naphthol (Jiangsu chiral chemical industry) and toluene and ethyl acetate successively. Mix 500mL of the solution (the mass ratio of toluene to ethyl acetate is 3:2), stir to dissolve it, and slowly add 53g (0.52mol) of sulfur dichloride dropwise under ice bath conditions, and control the temperature of the reaction system at about 15°C. After dripping, continue to react for 3h. The temperature was raised to 100°C, most of the solvent was distilled off, and then put into the refrigerator to freeze for 1 hour. Solids were precipitated, filtered, recrystallized from absolute ethanol, filtered, and dried to obtain bithionaphthol.
[0178] (2) In the reactor, add the dithionaphthol, sodium hydro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com