Group of plasma non-coding RNA, primer group for detecting expression level of plasma non-coding RNA and colorectal cancer (CRC) detection kit
A detection kit and technology for colorectal cancer, which are applied in the fields of genetic engineering and clinical medicine, can solve the problems that affect the diagnostic accuracy and positive rate of colorectal tumors, are not suitable for large-scale clinical use, and have low expression of non-coding RNAs. Achieve stable expression, easy detection, and accurate quantification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1 Research Object Selection and Grouping Basis
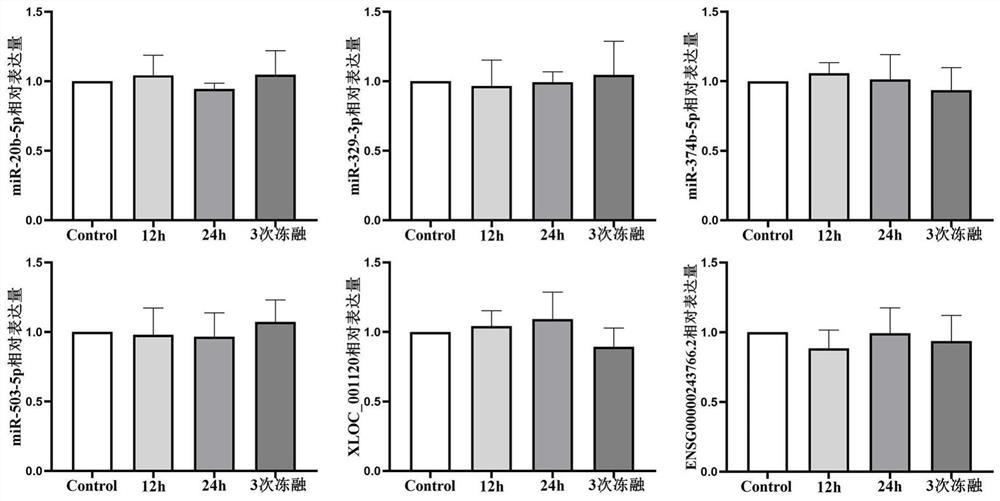
[0025] The purpose of this embodiment is to establish a unified specimen bank and database, collect standard blood samples with standard operating procedures, and systematically collect complete demographic and clinical data. In addition, in the same way, blood samples were taken from blood donors who were diagnosed with colorectal polyps and excluded from the diagnosis of colorectal cancer in the gastroenterology department during the same period, and blood samples were taken from those who underwent routine physical examination. All experiments were approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University. This study was conducted in accordance with the guidelines set by the Declaration of Helsinki.
[0026] Specific sample classification criteria are as follows:
[0027] Group A: Healthy control group (n=600, 3 persons for microarray screening, 597 persons for first-s...
Embodiment 2
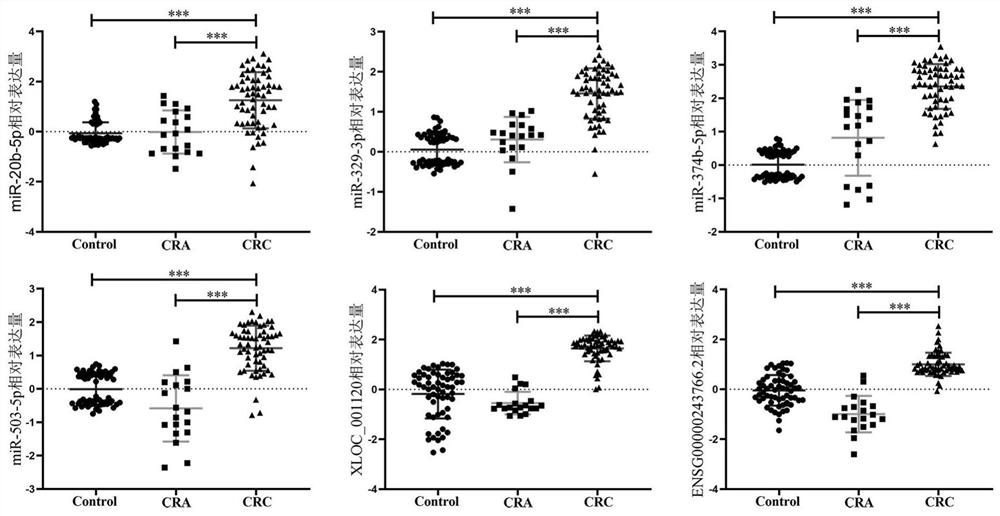
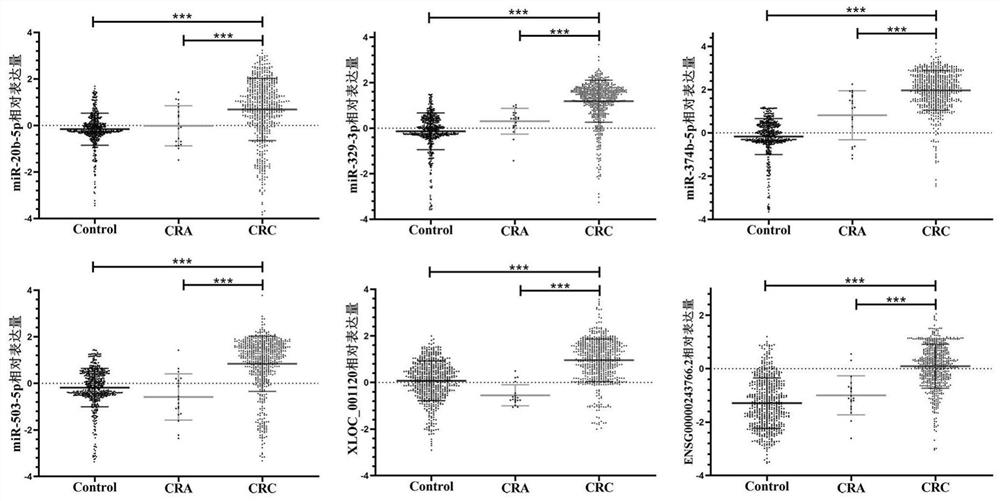
[0043] Example 2 Validation of Candidate Plasma miRNA Biomarkers
[0044] 1. Total RNA extraction and cDNA sample preparation:
[0045] The purpose of this example is to extract the RNA in the sample and prepare the cDNA required for subsequent experiments. The method used in this example is a conventional molecular biology method, and the reagents used are all commercially available (commodities / reagents). The steps of the specific example are as follows :
[0046] a) Take 100 microliters of plasma and add 5 milliliters of fresh heparin anticoagulant blood, centrifuge at 3000 rpm for 5 minutes in a centrifuge, and absorb the supernatant;
[0047] b) Add 900 microliters of Trizol reagent, oscillate and mix well, centrifuge at 12000 rpm for 15 minutes at 4 degrees, take the supernatant, and discard the waste liquid in the lower layer;
[0048] c) Add 1.5 times the volume of supernatant absolute ethanol, shake and mix, transfer to a spin column, centrifuge at 12,000 rpm for 15...
Embodiment 3
[0071] Example 3 Validation of Candidate Plasma lncRNA Biomarkers
[0072] The purpose of verification in this example is to verify the expression levels of the six lncRNA biomarkers screened in Example 1. Extraction of total RNA was as described in Example 2 above. The specific steps of cDNA required for reverse transcription follow-up experiments are as follows: take the pretreated plasma RNA, and obtain cDNA samples through RNA reverse transcription reaction. Prepare the required reaction system according to the instructions, as shown in Table 5 below:
[0073] table 5
[0074]
[0075] After the above system was mixed, it was centrifuged briefly, and the RT reaction program was: 42°C for 60 minutes, 70°C for 10 minutes.
[0076] 2. Detect the expression of plasma miRNA biomarkers obtained in Example 1, and verify the expression of plasma lncRNAs by RT-PCR method:
[0077] Prepare the qPCR reaction system on ice, as shown in Table 6 below:
[0078] Table 6
[0079]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com