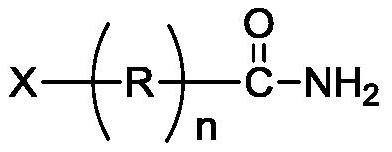
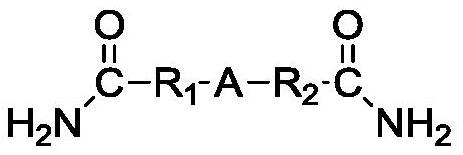Method for preparing 4-nitrosoaniline and 4-nitroaniline
A technology of nitrosoaniline and nitroaniline, which is applied in disproportionation to prepare amino compounds, amino substitution of hydrogen atoms, organic chemistry, etc., can solve the problem of low yield of 4-nitrosoaniline, achieve high selectivity, Effect of reducing pollution and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] First purging the empty autoclave with compressed air, then adding 7.44 grams of nitrobenzene, 120 grams of dimethyl sulfoxide, 2.2 grams of tetramethylammonium hydroxide pentahydrate, 10.6 grams of desiccant calcium oxide, and Trimethyl ammonium chloride 1.2 grams, then airtight autoclave, be filled with 1.63 grams of liquid ammonia, the pressure in the still reaches 1.6 MPa, and stirs 10 minutes under this pressure, records the volumetric concentration of ammonia in the gaseous phase in the still is 12%. The reaction mixture was heated to 80°C with stirring, and reacted at this temperature for 7 hours. After the reaction, the pressure still was released, cooled, the reaction mixture was weighed, and sampling was carried out by gas chromatography, and the calculated yield of 4-nitrosoaniline in terms of nitrobenzene was 56.4%, 4-nitroaniline The yield was 42.3%.
Embodiment 2
[0047] First purging the empty autoclave with compressed oxygen, then adding 7.44 grams of nitrobenzene, 240 grams of N,N-dimethylformamide, 1.94 grams of sodium methylate, and 50 grams of desiccant anhydrous magnesium sulfate into the autoclave, and then Airtight autoclave is filled with 4 grams of liquid ammonia, the pressure in the autoclave reaches 2 MPa, and it is stirred under this pressure for 10 minutes, and the volume concentration of ammonia in the gas phase in the autoclave is measured to be 85%. The reaction mixture was heated to 96°C with stirring, and reacted at this temperature for 7 hours. After the reaction, the autoclave was depressurized, cooled, and the reaction mixture was weighed and sampled for gas chromatographic analysis. Calculated in terms of nitrobenzene, the yield of 4-nitrosoaniline was 54.7%, 4-nitroaniline The yield was 43.7%.
Embodiment 3
[0049] First purging the empty autoclave with compressed oxygen, then adding 7.44 grams of nitrobenzene, 51 grams of N-methylpyrrolidone, 3.36 grams of potassium hydroxide, 63.6 grams of desiccant anhydrous sodium carbonate, tetramethyl bromide Ammonium chloride 3.5 grams, then airtight autoclave, be charged with 2.45 grams of liquid ammonia, the pressure in the still reaches 1.7 MPa, and stirs 10 minutes under this pressure, records the volume concentration of ammonia in the gas phase in the still and is 67%. The reaction mixture was heated to 100°C with stirring, and reacted at this temperature for 7 hours. After the reaction, the autoclave was depressurized, cooled, and the reaction mixture was weighed and sampled for gas chromatographic analysis. Calculated in terms of nitrobenzene, the yield of 4-nitrosoaniline was 54.7%, 4-nitroaniline The yield was 43.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com